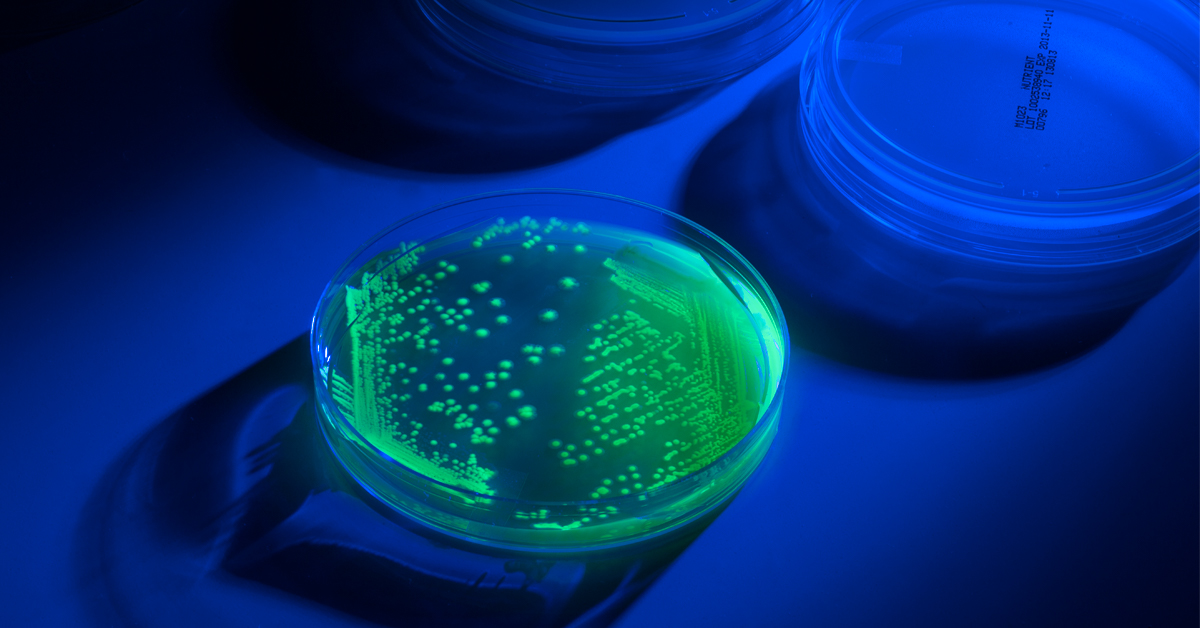
Operating within a narrow window of time for identifying pathogens, food safety microbiologists have the added uncertainty of cross-contamination from control strains, a common cause of false positives. The USDA Microbiology Laboratory Guidebook (MLG) and the FDA Bacteriological Analytical Manual (BAM) recommend the use of control strains with Green Florescent Protein (GFP) markers to clearly distinguish controls from naturally occurring microflora and true contamination. These strains fluoresce under UV light to eliminate all uncertainly of their origin. Microbiologics offers GPT strains under the UV-BioTAG line of controls for food QC. With UV-BioTAG, the GFP marker is integrated into the chromosome rather than the plasmid for enhanced stability. For insights into the day-to-day use of these control strains in the laboratory, we interviewed Dan DeMarco. Ph.D., Director of Science for Eurofins Microbiology Laboratories (EML). With over 800 laboratories in 50 countries, Eurofins brings extensive expertise in microbiology testing, including food safety.
How have your lab procedures changed since you began using GFP controls?
Nothing substantive has changed in our production labs. We have always and continue to stress the importance of including positive controls, as many in-house labs and quick TAT technologies do not. Positive controls are the only way to routinely verify that the methods being used by the lab are delivering the results we expect. We are still following best practices on training and sanitation as we always have. However, we now have much higher confidence because we can tell instantly when we are looking at the control strain.
What are the biggest benefits you see for GFP controls in your lab? What about UV-BioTAG™ specifically has made QC easier for you or improved your confidence in your processes?
The biggest benefits are speed and confidence in the result. Previously, though we used rare strains as daily process controls and they are highly unlikely to be found in nature, it cannot be ruled out with certainty; therefore one can never say for sure if a given strain arose naturally or was the result of a lab contamination event. Since GFP is not found in nature a positive result for its presence is absolute. Moreover, previously serology or molecular typing might have been required when troubleshooting possible control strain contamination. Those techniques can take hours or days; with UV it takes only minutes to get an answer (once you have a colony on a plate—see below for more on this).
What are some challenges of using GFP controls?
Two main challenges. The first, which is minimal and easily overcome with appropriate training, is that the result interpretation (presence of fluorescence) is made manually. A technician must be able to correctly identify that a given colony is fluorescing or not. Having the appropriate UV light excitation source and band pass glasses (if required) for visualization is critical.1 The second is inherent in the fact that you need a colony grown on plate in order to visualize the fluorescence. This takes time (at least 24 hours). In a contamination troubleshooting scenario every second counts. Fortunately, at least in the case of Salmonella Tranoroa (now available in UV-BioTAG format) Eurofins Technologies BACGene has developed a real time PCR assay specific for this widely used control strain. Now we have the ultimate one-two punch. A rapid, specific PCR method that can be used to test suspect contaminated enrichments directly, and a UV tag that can be used to test any colonies recovered from those enrichments.
What are some tips you recommend for laboratories to begin using GFP controls?
Make sure you understand that there are different GFP markers in the different UV-BioTAG strains. Understand what the appropriate light excitation source is and whether band pass glasses will be needed to visualize and make sure you have that equipment before starting. Do some training runs so that your technicians become familiar with the equipment and confident in identifying fluorescing colonies and have a good process in place for managing your controls to stay within the recommended maximum regrowths between reference and working cultures.
Is there anything else that you think would be important for labs to know regarding UV-BioTAG™ or GFP?
If you are not using these products, you should be. GFP labeling of positive control strains, especially in combination with specific PCR assays like the BACGene S. Tranoroa assay, is the best way currently available to get a rapid, definitive answer on the question of potential laboratory contamination. Eurofins always chooses best-in-class QC technology because we wish to give our customers confidence that we provide trustworthy results.
Green Fluorescent Protein and UV-BioTAG
To learn more about its history and use in reference materials, read our blog post on Green Fluorescent Protein. Check out the Microbiologics UV-BioTAG line to find the right GFP strains for your QC needs. 1. See Microbiologics UV-BioTAG documentation for additional UV equipment guidelines.
About Dan DeMarco
Daniel R. DeMarco, Ph.D. is currently the Director of Science for Eurofins Microbiology Laboratories (EML). In this role he functions as a technical lead for the EML research and development group managing a portfolio of projects focused on various new technology initiatives within EML and the broader US Food business. He is based in Louisville Kentucky where he also functions as the site’s resident internal/external microbiology/molecular expert in all areas related to food pathogen and food safety/quality indicator organism diagnostics. In both roles he consults extensively internally with the technical, sales, and marketing teams and with current and potential new customers acting as a technical resource as needed. Dr. DeMarco also chairs the EML technical committee, a cross BU/functional team of experts with extensive industry experience, that serves as a business wide resource for leadership at all levels, and rank and file employees for all technical issues of concern to EML.
Dr. DeMarco received his B.S. in microbiology from Ohio University in 1996. Following graduation he was awarded an Emerging Infectious Disease Fellowship with the Centers for Disease Control and Prevention. He spent the one year term of his fellowship in the enteric disease branch at the Massachusetts State Public Health Laboratory in Boston where he helped establish one of the earliest state satellite laboratories using pulse field gel electrophoresis and the precursor to PulseNet to track outbreaks of foodborne disease, including some of the earliest associated with E. coli O157:H7 in meat products.
Following his time with CDC, he entered graduate school at the University of South Florida where he received his Ph.D. in 2001 and was then recruited by DuPont to join their Central Research and Development division. He spent three years leading an industrial microbiology group in charge of preventing and controlling microbial contamination in industrial processes throughout DuPont’s various businesses. He also continued to pursue his interests in food microbiology and microbial detection technologies and in 2005 accepted a promotion into DuPont Qualicon as a senior research microbiologist. He was eventually promoted to project team leader and research manager in the newly named DuPont Nutrition and Health molecular diagnostics business where he led a group of Ph.D. level senior scientist developing end-point and real time PCR and reverse transcriptase PCR assays for various food pathogens including Listeria, Salmonella, and shiga-toxin producing Escherichia coli (STEC). He was the lead and/or co-lead research scientist on 8 successfully commercialized DuPont BAX® System assays including 24E Genus Listeria, 24E Listeria monocytogenes, STEC screening, STEC panel 1, STEC panel 2, Real-time Salmonella, Real-time Genus Listeria and Real-time Listeria monocytogenes assays. Shortly following their commercialization, the big-six STEC assays were adopted by USDA as an official rapid method in the USDA-MLG guidelines (USDA-MLG 5.09). Dan’s other major role in diagnostics at DuPont was in the evaluation of new technologies for detection and sample preparation in food pathogen testing applications.
Along with four issued US patents and multiple applications he has published in a number of peer reviewed technical journals and given posters and/or presentations as author/co-author and invited speaker at a host of food microbiology and detection technologies conferences across the globe. He also has extensive international experience and has spent considerable time consulting and serving as a technical resource on food microbiology topics (often focused on E. coli O157:H7/STEC testing) at industry and government sites around the world.
Dr. DeMarco is a prolific writer and has published >2500 articles on a variety of new media outlets. His writings (both serious and humorous) span a diverse array of topics including science, technology, business, philosophy, and short fiction to name just a few. Finally, Dr. DeMarco is an aspiring artist and creates whimsical and serious works of photomicroscopy which he markets under his personal brand, microbiology themed art like objects.




0 Comments