Pharmaceutical microbiologists have the great responsibility of ensuring their products are free of harmful contaminants and safe for consumers. Earlier this month at the Pharmaceutical Microbiology Fall Forum in Philadelphia, Microbiologics Vice President of Research and Development Brian Beck Ph.D. led a discussion on the importance of environmental isolate testing and identification in pharmaceutical laboratories.
Dr. Beck expanded on the how to identify isolates, next steps after identification, reasons laboratories should use their isolates for testing and methods available for preservation. The key points from Dr. Beck’s presentation below can help your laboratory implement reliable environmental isolate identification and preservation processes.
Importance of Environmental Isolate Testing
- Objectionable organisms are the primary reason for recalls. Some data show as much as 72 percent of recalls are caused by environmental isolates.1
- When environmental isolate testing is done correctly, manufacturers can have confidence in their finished products and protect their brands by avoid public product recalls.
- Identification should be performed on every contaminant to determine if it is objectionable.
- If the isolate is objectionable, the source of the contaminant should be investigated to determine the cause.
What does the FDA consider objectionable?
- Burkholderia cepacia
- Escherichia coli – indicator of fecal contamination
- Pseudomonas aeruginosa
- Salmonella species – pathogenic
- Shigella species
- Staphylococcus aureus – poor production hygiene
- If the manufacturing facility is sterile, all organisms are deemed objectionable.
You’ve identified an organism in your environment. Now what?
Environmental monitoring doesn’t stop at detection, but what are the correct next steps to keep your products safe and laboratory in compliance? Instances of U.S. Food and Drug Administration (FDA) warning letters and observations associated with inadequate environmental isolate testing are on the rise. As a result, laboratories are seeking guidance to determine what to do with their environmental isolates. These laboratories may consider including the isolates in disinfectant efficacy, growth promotion or antimicrobial effectiveness challenge tests.
Use the guide below, created in partnership with Scott Sutton, Ph.D., to determine the best plan of action the next time your laboratory discovers an environmental isolate.
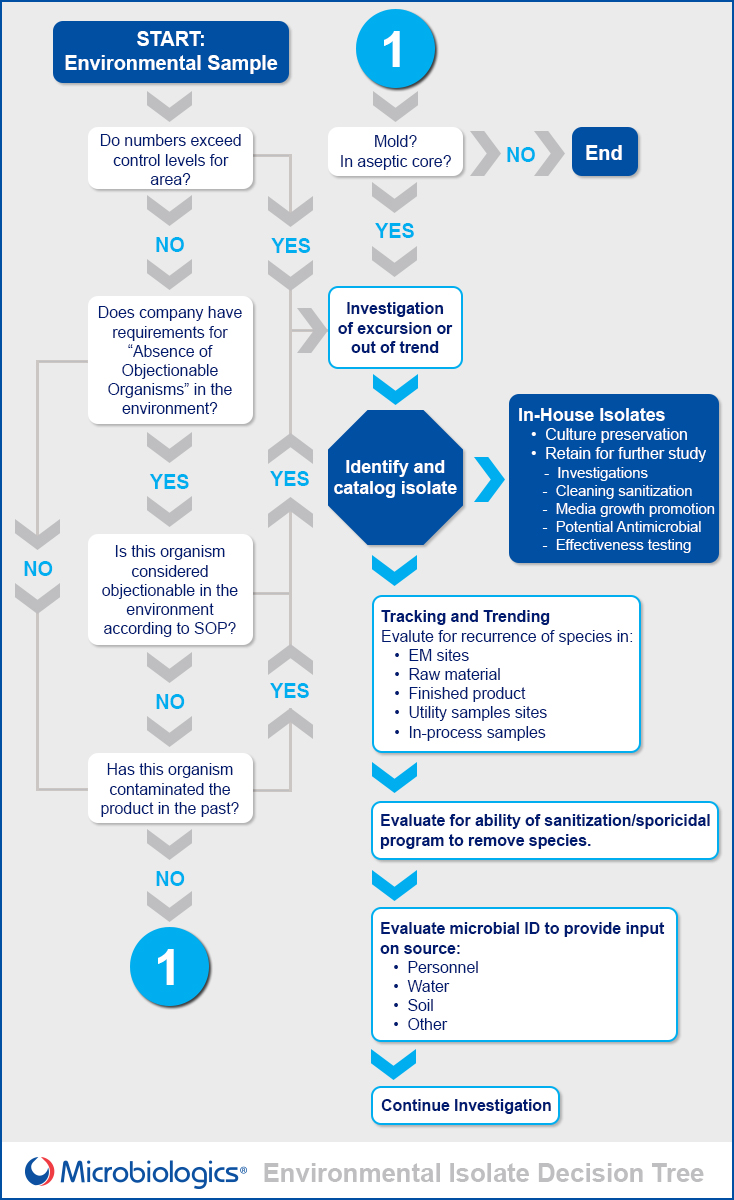
Identification
There are several ways to identify environmental isolates:
- Gram stain and cell morphology
- Identification to genus
- Identification to species
- Strain typing
Microbiology laboratories must balance cost and accuracy when deciding between the different testing schemes. Laboratories may choose to identify isolates using instrumentation systems or seek assistance from service providers who offer identification expertise.
Preservation and Use of Environmental Isolates
Your laboratory should preserve and test with its environmental isolates because:
- Wild strains differ from domesticated strains and have been known to be more resilient and hearty because they can adapt to their environment and nutrient supply.
- Tim Sandle stated in the Pharmaceutical Microbiology blog:
- “There is a strong argument that environmental isolates are the best challenge to media and for validation studies like sterility test validation. They are the most sensitive microorganisms, having become recently exposed to disinfectants, particular soils, etc.”2
- Environmental isolates may be more resistant to disinfectants as they adapt to the environment and limited nutrients.
- The isolates may need special growth conditions.
- When isolates contaminate a product, they can be difficult to detect.
- Effective tracking and trending analysis is essential:
- A proactive approach is always better than reactive.
- These data provide clues to the source of contamination.
Incorporate your isolates into your routine testing:
- Growth Promotion Testing
- USP 41 <1116> states “….[performed] to demonstrate that media used in the microbiological environmental monitoring program, or in media-fill runs, are capable of supporting growth of indicator microorganisms and of environmental isolates…”
- Consider all media used in your facility for environmental monitoring, media fills and sterility testing.
- Disinfectant Challenge Testing
- USP 41 <1072> states “…frequently isolated microorganisms from an environmental monitoring program may be periodically tested with the agents used in the disinfection program….”
- Select the right isolates for disinfectant testing:
- Provide rationale for the organisms used for your testing.
- Select well-known contaminants, organisms commonly isolated from the facility or strains that pose a particularly high risk to the products manufactured at the facility.
- Choosing the right organisms helps your laboratory avoid audit findings.
- Antimicrobial Effectiveness Testing
- Use recommended USP strains.
- Use environmental strains to measure preservative effectiveness.
- Products with extreme environmental conditions may warrant additional isolates capable of surviving these conditions.
Preservation Methods
Your laboratory can utilize several methods to preserve environmental isolates for on-going, routine testing:
- Sub-culturing/refrigeration. Although this method is low-tech and inexpensive, it also has the highest risk of bacterial mutation and contamination.
- Sub-zero Freezing (-20°C). Viability can be maintained for one to two years, but cellular damage can occur due to ice crystals and electrolyte fluctuations. This method carries a risk of contamination as well as additional costs for the freezer and maintenance.
- Ultra-low Freezing/Cryogenic Freezing. This method reduces the probability of mutation and offers a longer survival rate. However, the method is labor intensive, costly, requires close temperature monitoring and liquid nitrogen vapor poses safety concerns.
- Professional Preservation Services. Turn-key processes for isolate management including identification, manufacturing and storage. Advantages of this type of service include:
- Accreditation
- Experience
- Quality
- Finished goods storage
- Cost-savings
- Product warranty
- Test-ready formats
Key Takeaways
Every environmental isolate poses a risk to aseptic manufacturing. Accuracy identifying environmental isolates is crucial to consumer safety. Because current regulations require the use of isolates in testing, laboratories must develop long-term, sustainable environmental isolate programs.
If you’ve found an organism in your laboratory, Microbiologics can simplify your next steps. Send us your isolate for custom preservation and you’ll receive a QC testing kit designed for your laboratory with your isolate. Visit our website to learn more or fill out the form below for a consultation with our Custom Solutions team:
[ccf_form id=”5373″]
Resources
1 Sutton and Jimenez, American Pharmaceutical Review, Jan/Feb 2012
2 The Use of Environmental Isolates, Tim Sandle, Pharmaceutical Microbiology blog, January 10, 2010
Read Next – Growth Promotion Best Practices Auditors are Looking for in Pharmaceutical Labs





I am interested in those projets