When penicillin went into widespread use during WWII, it became known as “the wonder drug” for its revolutionary impact on infections. In addition to its ability to treat war wounds, it also dramatically changed treatment of common infections, including STIs like gonorrhea. First discovered in 1928, penicillin went through 15 years of unprecedented cross-Atlantic research and development before reaching large-scale application. Yet within less than five years of “the wonder drug” appearing on the frontlines of WWII, cases of penicillin resistance were documented. From the early years of penicillin usage, antibiotic development has been a race against time to bring new treatments to market before the existing drugs encounter pervasive resistance.
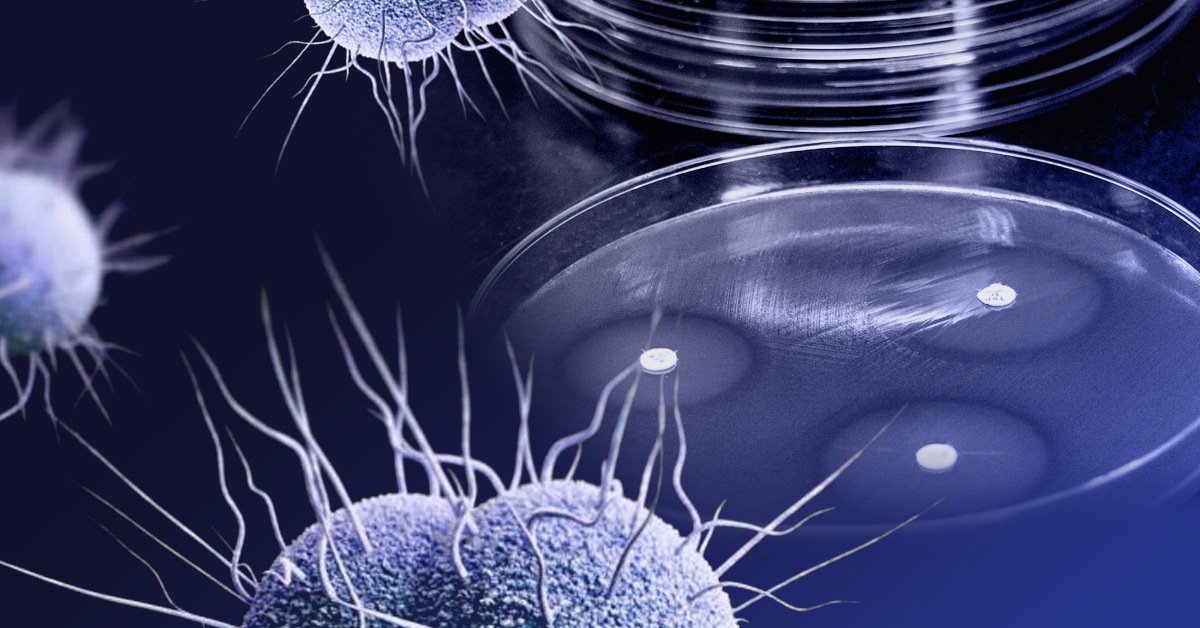
Perhaps no pathogen demonstrates this pattern of resistance better than the common STI, Neisseria gonorrhoeae. Although antibiotic resistance is common, Neisseria gonorrhoeae is notable for several factors, including its speed in developing antibiotic resistance mechanisms and its retention of resistance mechanisms.
With the introduction of penicillin, gonorrhea went from a life-altering infection to a mere nuisance that quickly disappeared for around 95% of treated patients. Ironically, the phenomenal success of “the wonder drug” would help contribute to the health crisis the world faces today. Many people commonly assumed that STIs were a problem of the past, a notion that persists today, as STI rates continue to rise after five straight years of double-digit growth. The first documented cases of resistance in gonorrhea appeared in 1946, just three years after large-scale adoption of penicillin. By the 1960s, doctors were reporting widespread failure of penicillin to treat gonorrhea. Each successive antibiotic approved for gonorrhea—tetracycline, spectinomycin, fluoroquinolones, macrolides, and cephalosporins—has followed the same pattern. After a few years of efficacy, resistance increases until the antibiotic must be replaced by newer, more effective treatments.
Now, after 80 years of antibiotics, the CDC warns that completely drug resistant—untreatable— gonorrhea may also soon be a threat. In 2010, the CDC adopted a regiment of ceftriaxone and cefixime, which are both within the cephalosporin class of antibiotics. Alongside these two antibiotics, the CDC also recommended the use azithromycin (belonging to the macrolide class of antibiotics) to minimize resistance to ceftriaxone. The United Kingdom and other countries implemented similar approaches. In 2020, the CDC again published new treatment recommendations to inhibit antibiotic resistance development, specifying the use of ceftriaxone alone. Based on gonorrhea’s history, ceftriaxone may have less than 10 years of remaining efficacy. No new treatments are close to approval for gonorrhea.
For gonorrhea, the development of antibiotic resistance poses unique challenges. Whereas most bacteria will lose antibiotic resistance once the antibiotic selective pressure disappears (when the drug is phased out of usage), Neisseria gonorrhoeae retains resistant properties even after prolonged absence of the antibiotic Therefore, antibiotics that were once effective but discontinued decades ago cannot be re-introduced into the portfolio of treatments, as they can be for other pathogens that develop resistance. Not only does Neisseria gonorrhoeae develop resistance quickly, but it also appears to be permanent in some cases.
On top of its unique ability to develop and maintain resistance, gonorrhea is also on the rise worldwide, along with other STIs. With the increased infection rates—and corresponding exposure to antibiotics—gonorrhea may develop resistance to remaining treatments even faster. Although gonorrhea is the extreme case of developing antibiotic resistance, it is far from the only example and continues to rise in part, because of lackadaisical attitudes toward these types of infections, resulting in mis-use of antibiotics, such as the failure to follow the complete treatment course. The declining public concern, in turn, also leads to decreasing budgets and lower testing rates. Early detection and proper antibiotic usage are critical for slowing the increase in resistance in not only Neisseria gonorrhoeae but other STIs as well.
Microbiologics QC Solutions
Microbiologics simplifies QC for molecular and culture-based diagnostics for women’s health and STIs. Designed to mimic the format of patient samples, Microbiologics controls are user-friendly, affordable, and reliable. Microbiologics offers controls for Human papillomavirus (HPV), Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and other common STIs.
Sources
“Antibiotic-Resistant Gonorrhea.” CDC. https://www.cdc.gov/std/gonorrhea/arg/default.htm
Dall, C. “Experts brace for more super-resistant gonorrhea.” Center for Infectious Disease Research and Policy. Sep. 04, 2018. https://www.cidrap.umn.edu/news-perspective/2018/09/experts-brace-more-super-resistant-gonorrhea
Gaynes, R. (2017). The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerging Infectious Diseases, 23(5), 849-853. https://doi.org/10.3201/eid2305.161556
Meyerowitz, E. A., MD, and Goldstein, R., MD, PhD. (2019) “Sexually transmitted infections are on the rise: Should you worry?” Harvard Health Blog. https://www.health.harvard.edu/blog/sexually-transmitted-infections-are-on-the-rise-should-you-worry-2019121118370
“Multi-drug resistant gonorrhoea.” WHO. https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea
“STDs at record high for 6th year in a row.” (2021) Medical Laboratory Observer (MLO). https://www.mlo-online.com/disease/stis-stds/article/21218676/stds-at-record-high-for-6th-year-in-a-row
“Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020.” CDC. Dec. 18, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6950a6.htm




0 Comments