The Drawbacks of Using Frozen Cultures in Clinical Diagnostics
Maintaining bacterial stocks via frozen methods is a common way to preserve viable cultures. Frozen stocks may appear to be cost effective and user friendly, but freezing and thawing can create several problems. Some of these issues include:
- Time Consumption
- Cost Ineffectiveness
- Contamination
- Reduced Recovery
- Loss of Genetic Material & Expression
Despite these drawbacks, many laboratories continue to use frozen stocks, which may void product warranties and may compromise quality. Let’s discuss some of these concerns in further detail.
Time Consumption
It’s a common misconception that using frozen stock cultures is a time saving measure, when in fact it can be labor-intensive. There are two processes required for maintaining cultures: (1) The preparation of stocks and (2) the use of those stocks.
Preparing Stocks
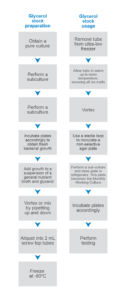
Preparing frozen cultures requires many steps, such as a sub-culture, transferring growth and other required components into a suspension for freezing, and aliquoting the suspension into cryovials. A highly viscous solution that does not pipette well, such as glycerol, may require additional time to ensure an appropriate volume has been added. Using commercially prepared cryovials or beads that arrive with the suspension may save some time and resources, but you may be limited in the number of bacterial cells you can add with those cryovials1, or the bacterial cells will experience numerous freeze thaw cycles2, which is also detrimental as the number of cycles accrue.
Stock preparation flowchart (click to enlarge)*
* Actual procedures may differ and may be more extensive.
Using Stocks
Once a new culture is needed, it can take several minutes to obtain a fully thawed suspension. A suspension must be completely thawed to ensure the suspension is thoroughly mixed, as any remaining frozen stock could have bacterial cells that clumped together when freezing. Some commercially available cryogenic systems require the top to be scraped1 or one bead to be removed while frozen to inoculate the plate; however, this process could result in no recovery, since the cells may still be in a preserved state.
In addition to preparing frozen stock and stock culture for recovery, technicians may need to go back to the frozen stock to regrow or retest if failures occur. The strain’s special characteristics, such as antibiotic resistance, may be lost if the culture is frozen (see below). Besides the obvious problems associated with a time-consuming procedure, a time-intensive task can exacerbate other problems, especially if it must be repeated often. A multistep process may become prone to errors if it places time constraints on already strained laboratory resources.
Cost Ineffectiveness
With pressure to keep operating costs low, using frozen stocks appears to be a great solution. You can freeze a culture of bacteria onto beads or in glycerol stock aliquots and use it for years! All you need is an ultra-low freezer. Ultra-low freezers are expensive themselves, but there are the additional costs of maintaining and operating an ultra-low freezer.3 It is estimated that for one day, one ultra-low temperature freezer uses the same amount of energy as the average household.4,5 Even if your laboratory has an ultra-low freezer, the use of it for reference cultures may increase its ongoing costs.
Contamination
Contamination may occur at every step during preparation and even after. Many protocols state to use growth from a liquid culture; however, there’s no way to visually confirm absence of contamination.7,8 Each time you handle a frozen culture may introduce contaminants. As usage increases, it can become harder to identify where and when a contaminant could have entered the cryovial. If contamination is found, a second attempt from that same cryovial may be attempted, or that cryovial may be discarded and a new cryovial used. Additionally, if snap cap microcentrifuge tubes are used, the caps may pop open at -80°C, which may contaminate surrounding test tubes. The presence of contamination requires additional resources and technician time.
Reduced Recovery
Perhaps the biggest concern for using frozen stock is that many downstream applications can be affected, from recovery to genetic mutations (see below). Recovery of frozen stock can be affected by the growth stage used, number of freeze-thaw cycles, the components used in the cryopreservation solution, and even the type of bacterium. Some species, especially fastidious organisms, may not recover well from a frozen state. Currently, there is no standard or recommended expiration date for frozen cultures, and different sources state different lengths of time. Guidelines range from “reasonable amount of time” up to several decades. 1, 2, 8, 9, 10 The cryoprotectant used can also affect the length of time for which the culture is viable, even in a frozen state.11 Months or years of testing may be needed to validate freezing methods, but even then, these methods may not work for all strains. Freezing methods are not always ‘one-size fits all’ solutions that meet the requirements of fastidious and anaerobic organisms.
Many cryopreservation methods found online state to use cells from the log phase;7 however, some studies suggest that cells in the stationary phase may be best to use. Cells in log phase may be more susceptible to rapid chilling due to ice formations compared to cells in stationary phase, which are insensitive to this method.12 The risk with using stationary phase growth, however, is that you could wait too long and unknowingly use cells that are no longer viable. Gram positive and Gram negative cell types may be differently affected by cryopreservation. The cell walls and presence of peptidoglycan are beneficial for Gram positive cells as they help prevent damage from physical stock. In comparison, Gram negative cells are more susceptible to dehydration and cell membrane damage.11 This one difference highlights the difficulty of creating a universal preservation method, and doesn’t take into consideration many other factors, such as variations between species or strains within the same species.
Multiple freeze-thaw cycles affect recovery by allowing cells to expand and shrink quickly between each freeze-thaw cycle, resulting in ruptured cell membranes. Cells can become mechanically damaged by ice crystals, but also chemically damaged as salts and proteins become concentrated with each freeze-thaw cycle.13 One study on the impacts of freezing and thawing observed that Limosilactobacillus fermenti cells experienced the greatest loss with the second freeze compared to the first freeze or subsequent freezes.14
Loss of Genetic Material
Another common disadvantage to cryopreservation is the opportunity for genetic mutations to occur, often resulting in known characteristics to be lost. Klebsiella pneumoniae K-9 has demonstrated unencapsulation from capsulation at high frequencies and also cellular injuries after freezing.15 A study of the effects of freezing on E. coli found that 1% of rare alleles were lost. Since E. coli is a stable, hardy species, the results of this study would indicate the problem to be widespread and significantly worse for fastidious species.
The genes that provide antibiotic resistance are commonly found on plasmids, which may be lost during cell division, due to the cell’s fitness cost, or even in environments with lower antimicrobial levels. When a plasmid is rejected from a cell, a strain that once had antibiotic resistance is now susceptible. Enterococcus faecalis ATCC 51299 is commonly recommended as a QC organism for high level gentamicin resistance assays; however, this strain is notorious for losing the plasmid containing antibiotic resistance when stored improperly under freezing conditions. While the technology needed to perform these evolutionary studies on frozen cultures has come a long way and is more readily available, studies regarding how freezing affects cultures are not commonly performed due to the length of time required (often requiring multiple years) and high costs to perform these studies to observe changes in cultures.
A resource-intensive process with higher error rates
Given the factors above, the drawbacks of using frozen stocks often outweigh any benefits. Using frozen stocks may cost more and consume more time, in addition to the increased risks of contamination, reduced recovery, and loss of genetic material & expression. The use of ready-to-use control strains may avoid these drawbacks.
Maintenance of Quality Control Strains With Microbiologics
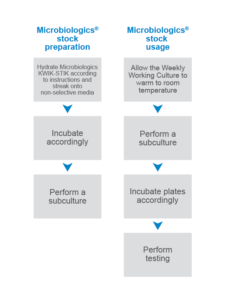
Microbiologics has been helping laboratories skip the freezer for reference strains for decades. The Microbiologics KWIK-STIK™ has everything you need for simple, reliable QC of culture-based methods: a lyophilized microorganism pellet, hydrating fluid, and inoculating swab. By following Microbiologics maintenance plan, no ultra-low temperature freezer is required as both product and culture are stored at 2-8°C. Fewer steps required for maintenance mean fewer chances for error, contamination, and mutations. KWIK-STIK’s simple, all-inclusive design optimizes accuracy and efficiency.
Preparing & using stocks with Microbiologics (click to enlarge)
With strains from Acetobacter aceti to Zygosaccharomyces rouxii, we’ve got over 900 reasons to choose KWIK-STIK™ for your testing needs. Available in packs of 2, 6, or as a QC set, KWIK-STIKs are commonly used for all types of microbiological control testing.
References
- Key Scientific Products, Inc. (2024). CryoCare bacterial preservation.
- Smith, D., Ryan, M. (2012). Implementing best practices and validation of cryopreservation techniques for microorganisms. The Scientific World Journal, 2012.
- Jana, A.M., Singh, P. (2020). Bacterial preservation. International Journal of Life Sciences and Technology, 13(1): 1.29.
- Air Products. (2024). The cost of mechanical ultra-low temperature freezers. One Nucleus.
- Faugeroux, D. (2016). Ultra-low temperature freezer performance and energy use tests. University of California, Riverside.
- Addgene. (2024). Creating bacterial glycerol stocks for long-term storage of plasmids. Protocols.
- QIAGEN. (2024). Working with DNA: Good microbiological practice. General Guidelines for Handling DNA.
- Newsom, E., Moffat, M., Acheampong, A., Zhang, S., Onadipe, A. (2017). Improving microbial cryopreservation methods. American Pharmaceutical Review.
- Sunarno, Nursofiah, S., Hartoyo, Y., Amalia, N., Febrianti, T., Febriyana, D., et. al. (2021). Long-term storage of bacterial isolates by using tryptic soy broth with 15% glycerol in the deep freezer (-70 to -80°C). IOP Conference Series: Earth and Environmental Science, 913.
- BiteSizeBio. (2023). How to preserve microorganisms: store your cells better. Cells and Model Organisms.
- Guo, N., Wei, Q., Xu, Y. (2020). Optimization of cryopreservation of pathogenic microbial strains. Journal of Biosafety and Biosecurity, 2: 66–70.
- Mazur, P. (1963). Physical-chemical basis of injury from intracellular freezing in yeast. Cellular Injury and Resistance in Freezing Organisms : proceedings, 2:171-189.
- BiteSizeBio. (2014). Freeze-thaw cycles and why we shouldn’t do it. Basic Lab Skills and Know-how.
- Harrison, A. (1955). Survival of bacteria upon repeated freezing and thawing. Vanderbilt University: 711-715.
- Takahashi, M., Ito, S., Yoshida, K. (1982). Effects of freezing and thawing and storage by freezing on the biological and morphological properties of strain K-9 of Klebsiella pneumoniae and its variants. Journal of Basic Microbiology, 22(9): 649-660.
- Sprouffske, K., Aguilar-Rodriguez, J., Wagner, A. (2016). How archiving by freezing affects the genome-scale diversity of Escherichia coli populations. Genome Biol. Evol. 8(5):1290–1298.






0 Comments