Last month we outlined the debate for and against requiring B. cepacia testing for all nonsterile pharmaceutical, personal care products and water systems (The Great Debate Over B. cepacia Testing). At the end of the post, we asked our readers what they think – should testing be required or not?
The Results Are In
We heard from over 100 readers, and their opinions were split:
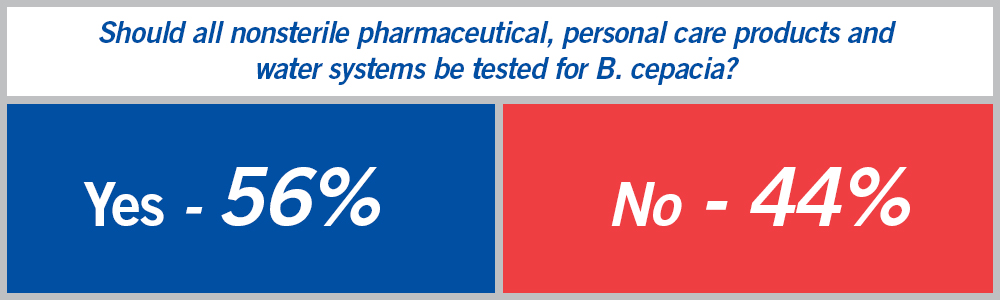
The poll results show that the B. cepacia testing debate among pharmaceutical and personal care product lab professionals will go on. However, there was an interesting development just a few days after we shared our Great Debate Over B. cepacia Testing post.
On May 22, the U.S. Food and Drug Administration (FDA) alerted drug manufacturers of a recent spate of product recalls due to contamination with Burkholderia cepacia complex. The contamination was seen in nonsterile, water-based products.
FDA Recommendations
The FDA made several recommendations for preventing objectionable microorganisms from contaminating established products. Four recommendations are:
1. Prevent contamination in the first place
Establish procedures to prevent objectionable microorganisms from contaminating nonsterile products. Examples of procedures include monitoring of environmental conditions and assuring adequate quality of incoming materials. See Sec. CFR 211.113 (a).
2. Use scientifically sound and appropriate acceptance criteria
Use scientifically sound and appropriate acceptance criteria to assure that drug product components (including pharmaceutical water) and finished drug products conform to appropriate quality standards. Criteria can be found in:
- USP Chapter <1111> Microbiological Examination of Non-sterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use
- USP <61> Microbiological Examination of Non-sterile Products: Microbial Enumeration Tests
- USP <62>Tests for Specified Microorganisms
Laboratory controls shall include the establishment of scientifically sound and appropriate specifications, standards, sampling plans, and test procedures designed to assure that components, drug product containers, closures, in-process materials, labeling, and drug products conform to appropriate standards of identity, strength, quality, and purity. See CFR Sec. 211.160 (b).
3. Use validated test methods
Ensure that the methods used to test finished drug products prior to release for distribution are appropriately validated, accurate, sensitive, specific and reproducible. For example, there shall be appropriate laboratory testing, as necessary, of each batch of drug product required to be free of objectionable microorganisms. See CFR Sec. 211.165.
4. Test in-process materials
Test in-process materials during the production process (e.g. at commencement and completion) using valid in-process specifications to assure, among other things, that the drug product will meet its final specification, including criteria for absence of microbial contamination, where appropriate.
To assure batch uniformity and integrity of drug products, written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch. Control procedures shall include bioburden testing. See CFR Sec. 211.110.
Here’s some good news for pharmaceutical and personal care product laboratories – B cepacia is now available in
EZ-Accu Shot™ for Growth Promotion Testing! Visit our website to find the QC microorganisms your lab needs in a variety of easy-to-use formats.
Reference
U.S. FDA. FDA advises drug manufacturers that Burkholderia cepacia complex poses a contamination risk in non-sterile, water-based drug products. 5/22/2017






APIs should be tested (every tenth batch) for B.cepacia before release.