By Marylou Gibson, Ph.D
Adapted from a post that originally appeared on LinkedIn.
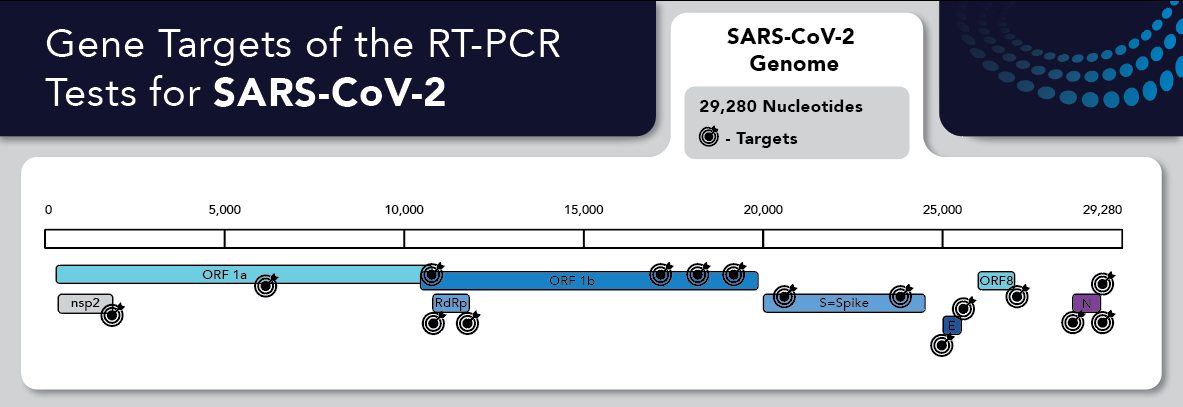
Of the more than 55 RT-PCR Test kits or assays currently available under Emergency Use Authorizations (EUA) by the FDA, few target the same genes. Many more similar PCR based tests are available outside the US. Manufacturers have performed analyses on the novel SARS-CoV-2 virus as compared to previous versions of human and animal derived coronaviruses to determine unique genomic targets.
| Diagnostic Test Name (Letter of Authorization) | Gene Targets | ||
| CDC 2019-n-CoV Real-Time RT-PCR Diagnostic Panel (CDC) | N1 | N2 | N3 |
| New York Department of Health, SARS-CoV-2 Real-time Reverse Transcriptase (RT)-PCR Diagnostic Panel | N | N | |
| Roche Molecular System Cobas SARS-CoV-2 | ORF1ab | E | |
| Thermo Fisher TagPath COVID-19 Combo Kit | ORF1ab | N | S |
| LabCorp COVID-19 RT-PCR Test | N | ||
| Hologic Panther Fusion SARS-CoV-2 | ORF1ab | ORF1ab | |
| Quest SARS-CoV-2 rRT-PCR | N1 | N3 | |
| Quidel Lyra SARS-CoV-2 Assay | ORF1ab | ||
| Abbot RealTime SARS-CoV-2 assay | N | RdRp | |
| GenMark ePlex SARS-CoV-2 Test | N | ||
| DiaSorin Simplexa COVID-19 Direct Kit | ORF1ab | ||
| Cepheid Xpert Xpress SARS-CoV-2 test | N1 | E | |
| Primerdesign Ltd COVID-19 genesig Real-Time PCR assay | ORF1ab | ORF1ab | |
| Mesa Biotech Accula SARS-CoV-2 Test | N | ||
| BioFire COVID-19 Test | ORF1ab | ORF1ab | OrF8 |
| PerkinElmer New Coronavirus Nucleic Acid Detection Kit | N | ORF1ab | |
| AvellinoCov2 test | N1 | N3 | |
| BGI Genimics Real-Time Fluorescent RT-PCR Kit for Detecting SARS-2-10-nCoV | ORF1ab | ||
| Luminex NxTAG CoV Extended Panel Assay | N | ORF1ab | E |
| Abbot Diagnostics ID Now COVID-19 | RdRp | ||
| Qiagen QiAstat-Dx Respiratory SARS-CoV-2 Panel | RdRp | E | |
| NeuMoDx SARS-CoV-2 Assay | N | nsp2 | |
| Ipsum Diagnostics COV-19 IDx assay | Unknown | ||
| BD BioGX SARS-CoV-2 Reagents for BD MAX System | N1 | N2 | |
| Co-Diagnostics Logix Smart Coronavirus Disease 2019 (COVID-19) Kit | RdRP | ||
| ScienceCell SARS-CoV-2 Coronavirus Real-time RT-PCR (RT-qPCR) Detection Kit | N1 | N2 | N3 |
| Luminex ARIES SARS-CoV-2 Assay | N | ORF1ab | |
| BD SARS-CoV-2 Reagents for BD MAX System | N1 | N2 | |
| KorvaLabs Curative-Korva SARS-CoV-2 Assay | N1 | N2 | |
* Microbiologics provides this information in good faith as a resource to the diagnostic community. If any information is incorrect or incomplete, please contact us with corrections.
Typically, assay manufacturers do not divulge the exact sequence of their primer/probe sets in their Instructions For Use (IFU) and usually only cite the gene. The ORF1B gene contains about 10,000 bases, a vast area suitable for probe design. Even the N gene is ~1300 bases and contains many unique targets. Test manufacturers choose different target sequences for PCR testing for several reasons, including the need for sequences in proven stable parts of the genome not likely to mutate.
Benefits & Drawbacks
The range of PCR targets has several benefits and drawbacks. One benefit is the ability of tests to remain accurate even if a target sequence mutates, rendering a specific probe useless to detect SARS-CoV-2. That is why manufacturers often include secondary and sometimes tertiary sites in their assays as a failsafe. If one target in an assay produces a false negative due to mutations, the second and third sites can generate PCR signals to correctly identify the presence of SARS-CoV-2. Mutations in two or three genome regions is unlikely. The plurality of tests provides the same kind of failsafe industry wide. If one assay fails to detect SARS-CoV-2 due to mutations, another assay with different targets could correctly identify its presence. Although this type of testing redundancy is rare on a per-patient level, it gives labs the flexibility to adapt to the unforeseen. Additionally, if one assay proves to have a higher than acceptable rate of error, labs can adopt other assays.
The largest drawback to this plurality is user confusion and information overload. Selection and use of the proper subgenomic synthetic RNA positive control is even more critical with SARS-CoV-2, given the speed at which assays entered the market. The target information for most assays is not usually transparent, and the user must search for them in the IFU or other documents. Plus, a suitable control is not always evident based on genes listed in the IFU. For example, the ORF1ab gene is 10,000 nucleotides and too large to be easily cloned or expressed as synthetic RNA for use as a control.
Better Control Solutions for Diverse Gene Targets
A more comprehensive solution is to use a whole genome RNA or, even better, a killed virus containing the entire genome. A killed virus control can be used as a full process control and is the most similar to an actual patient sample. Better controls are rapidly becoming available as labs ramp up production of tailored products.
SARS-CoV-2 Controls from Microbiologics
Microbiologics offers IVD and RUO controls featuring diagnostically relevant large RNA molecules for the RdRp/Orf1ab, S, E, and N genes. Learn more.





Hello,
The information about Testing and their targets is very helpful to avoid the spread of covid 19
The benefits and drawbacks of performing test is well explained.
Thank you.
This was exactly the info I was looking for- so thank you! I am wondering when the list of PCR targets of tests being used in the US was last updated.