This post addresses a new paradigm in Human Papillomavirus (HPV) diagnostic testing, moving away from cytological evaluation to primary HPV testing and offers guidelines for implementing this shift in your laboratory. We will describe US and global clinical testing guidelines regarding HPV and its value in identifying early cervical cancer. You will gain insight to the optimal quality control materials for HPV laboratory evaluation and learn about HPV self-collection as a promising method for patient screening.
Human Papillomavirus (HPV) is a small, non-enveloped, double-stranded DNA virus with over 200 identified genotypes. Some genotypes have adverse effects on health and cause serious symptoms such as genital warts and cancers, while others are asymptomatic. Forty of these HPV types are capable of infecting the human anogenital mucosa.1 Among the human-associated types, fourteen HPV serotypes have been linked to development of cervical cancer and/or precancerous lesions. Focusing on these more, the Centers for Disease Control and Prevention (CDC) recognizes HPV types 16 and 18 as the highest risk and lists additional high-risk types (31, 33, 45, 52, and 58) as being oncogenic.2
Detection of HPV infection is essential as the global epidemiology of HPV is changing due to the prevalence of varying serotypes. In the US and EU, HPV 16 and 18 are the predominant types, whereas in Africa, HPV type 52 predominates.3 Additionally, a patient may harbor multiple HPV types simultaneously. Therefore, a diagnostic test that targets more than just one HPV genotype is necessary to ensure the best patient screening and accuracy for viral detection in diverse populations.
Primary Testing for HPV vs. Co-Testing
Primary testing refers to using a single test for HPV, whereas co-testing indicates use of both a single test in conjunction with a Pap (Papanicolaou) test. Reflex testing is another approach where a HPV primary test occurs first, then if positive, a Pap test follows. To address current testing needs for HPV, let us first explore some recent laboratory trends in cytology:
| Testing Strategy | What’s Tested First? | Follow-Up Action | Common Use Case |
| Primary HPV Test | HPV only | Reflex cytology if positive | Most guideline-recommended approach |
| Co-Testing | HPV + Cytology together | Both results guide decisions | Widely used in U.S. women 30+ |
| Reflex Testing | Cytology first | HPV if Pap is abnormal | Older or traditional protocols |
The cytology laboratory had been traditionally used as the standard of care for patient HPV screening via the Pap smear. However, a new trend has emerged in the use of Artificial Intelligence (AI) as an aid for the pathologist in the identification of HPV from liquid-based cytology (LBC) slides. Use of AI has yielded greater diagnostic accuracy, specificity, and sensitivity and decreased interobserver variability than traditional pathologist evaluation. In fact, a recent study showed that use of AI-assisted cytology screening has the potential to be widely used as a primary strategy for cervical carcinoma.4 What about the core clinical laboratory? With the most recent advances, is there now a fundamental change in the actual screening population due to HPV vaccination?
Let us first assess where in the lab you currently test for HPV: in the cytology, microbiology, or the molecular laboratory. If HPV testing is performed in the microbiology or molecular laboratory, then the clinical specimen will be initially routed there, not to cytology. Thus, when moving away from the traditional Pap analysis (cytology) to a primary HPV test, it is necessary to get all personnel trained, especially those working in specimen accession/procurement and the individuals who actually perform the test.
Benefits of primary HPV testing for the patient population
According to a recent article in CAP TODAY5, a shift away from PAP testing to primary HPV testing offers several benefits, including a decreased need for patient specimen recollection. In addition, primary HPV screening may be a better method than the traditional Pap, as the prevalence of patient precancerous lesions is changing due to HPV vaccination.6 Thus, the focus is now shifting to use of primary HPV testing via molecular and laboratories must identify the best way for its implementation.
Regulatory guidelines and the shifts in HPV screening
The changes in testing methods raise several questions about regulations. How do current regulatory guidelines address co-testing using both primary and Pap testing? Along with changing diagnostic technology, what kind of HPV quality control materials are needed for this paradigm shift? Let us have a closer look at these topics. Regulatory agencies recognize the need for detection of a broader range of HPV types, including those of the high-risk group. In the US, an excellent resource is available from the American Society for Colposcopy and Cervical Pathology (ASCCP)7 and reinforces the need for primary HPV screening and reflex cytology for all positive HPV tests; see below.
ASCCP screening guidelines for HPV
The following recommendations are adapted from the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors.7
With primary HPV screening, the laboratory should perform additional reflect triage testing (e.g., reflex cytology) for all positive HPV tests regardless of genotype, including positive tests for genotypes HPV 16/18.
If primary HPV screening yields positive results for HPV 16 or HPV 18, but reflex triage testing from the same laboratory specimen is not feasible, the patient may be referred for colposcopy prior to additional testing.
If tests are positive for HPV 16 or 18, and triage testing has not been performed prior to the colposcopy, an additional sample should be collected for testing during the colposcopy visit.
Positive results for HPV-16 and HPV-18 indicate the highest risks for CIN 3, a precancerous condition of the cervix, and occult cancers; therefore, additional testing procedures should be performed following all positive results for HPV-16 and HPV-18.
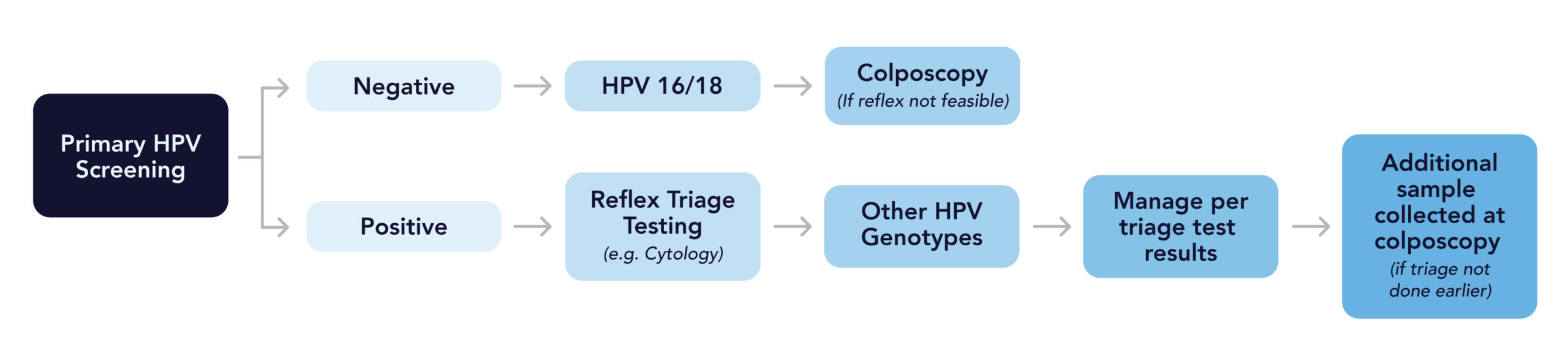
Other HPV cervical cancer screening recommendations are available from the US Preventative Services Task Force.8
WHO screening and treatment recommendations
Globally, the WHO9 has identified a strategy to accelerate the elimination of cervical cancer as a public health problem and has issued the following recommendation for primary HPV screening.
For the general population and for women living with HIV, the WHO recommends using HPV DNA detection for primary screening, rather than VIA or cytology.
Deciding on the best primary HPV test platform
The American Cancer Society (ACS) has provided flowcharts to assist in the process of choosing a platform. These flowcharts are based on the current ACS guidelines and give US-based laboratories choices on FDA-cleared, HPV testing platforms. Among those cited include the HPV tests/platforms from Abbott Molecular, BD, and Roche. For those laboratories outside the US, a good HPV reference is available from the WHO.
The latest trends in HPV primary testing
The newest trend is vaginal self-sampling for HPV. Self-sampling represents a new, positive step forward to elimination of cervical cancer, a strategic goal of the WHO.10 Basically, a patient uses a vaginal swab to collect cells from the vaginal area, rather than the cervix. Self-sampling has proven to be a convenient and less invasive alternative and shows comparable accuracy (>95%) to clinician-collected samples for high-risk HPV detection.11 It offers compatibility and acceptability among women from different geographies and income backgrounds. To address this new sampling methodology, a recent guideline has been issued on recommendations for the use of self-collected vaginal specimens for HPV testing in health care settings.12 Once implemented, there is the potential that using HPV self-collection may show a long-term predictive decrease in cervical cancer for patients.
Regardless of the HPV test/platform chosen, if your laboratory’s choice is primary testing using molecular assays, it is imperative that your laboratory uses the appropriate quality control (QC) materials. This should include a QC option with broad coverage of the HPV genotypes detected in the clinical analysis. Today’s commercial molecular tests offer coverage of the main HPV high-risk types 16 and 18, with the others (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) combined in most pooled test panels. Therefore, when choosing a QC vendor, genotype coverage is extremely important.
The significance of genotype variability
There are oncogenic risk differences among the various HPV genotypes. As we know, HPV 16 and 18 are high-risk types and are responsible for almost 70% of cervical cancers. Other genotypes such as HPV 6 and 11 are low-risk and usually linked to benign genital warts.2 HPV genotypes also matter for vaccine efficacy assessment. Most HPV vaccines target specific genotypes. See Table below for HPV vaccine specific targets.
| HPV Vaccine | Genotypes | Manufacturer |
| Gardasil® 9 | 6,11,16,18,31,33,45,52,58 | Merck & Co,, Inc. |
| Gardasil® | 6,11,16,18 | Merck & Co,, Inc. |
| Cerarix® | 16,18 | GlaxoSmithKline |
Genotype variability also is important for monitoring epidemiological trends in populations. In fact, due to the high vaccination rates in the EU and in North America, HPV 16 and 18 prevalence is on the decline, while other genotypes are becoming more predominant. In Sub-Saharan Africa, HPV 35, 52, and 58 are more common than in many Western countries.3
Another important goal for selecting your QC is to ensure that it is in line with your analytical processing steps. That is, choose QC materials that follow, along with the clinical specimen, the steps of cell lysis, nucleic acid isolation/purification, and target-specific detection. Other important factors are the QC material’s compatibility with the current patient testing workflow, laboratory instrumentation, and their long-term utilization efficacy.
Choosing the right QC partner for primary HPV screening
As laboratories shift toward primary, genotype-based HPV testing, robust and forward-thinking controls are essential. Microbiologics supports this evolution with rigorously engineered IVD products developed under FDA and IVDR design-control standards. The new Comprehensive HPV Control Panel is an independent, third-party control built for today’s high-throughput molecular workflows. Our control supports the increased adoption of molecular methods for diagnostic testing and the increased speed, throughput, and accuracy they provide. Covering 14 high-risk HPV types, it delivers IVD quality to simplify accreditation and remains relevant as screening guidelines advance. Find a full list of Microbiologics Women’s Health and STI controls here.
Guest Author:
 |
Andrea Pierce, PhD, MBAAmpSci Consulting LLC |
Andrea is experienced in Medical & Scientific Affairs for the clinical diagnostics industry. She has developed FDA 510(k) and IVDR compliant Instruments and PCR-based assays. Andrea has served as the Subject Matter Expert for New Product Development and Quality teams related to FDA 21 CFR 820 and ISO 13485 standards, and is recognized for leadership in regulatory compliance, international market expansion, and clinical affairs.
Sources
- Walboomeers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189(1): 12-19.
- CDC; https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-11-human-papillomavirus.html#cdc_report_pub_study_section_1-overview; [Accessed April 9, 2025]
- Osmani V, Rossiter M, Hörner L, et al. Worldwide burden of cervical human papillomavirus (HPV) in women over 50 years with abnormal cytology: a systematic review and metanalysis. BMJ Glob Health 2025;10:e017309. doi:10.1136/ bmjgh-2024-01730 https://pmc.ncbi.nlm.nih.gov/articles/PMC11966958/pdf/bmjgh-10-4.pdf
- Yang, W., Jin, X., Huang, L. et al.Clinical evaluation of an artificial intelligence-assisted cytological system among screening strategies for a cervical cancer high-risk population. BMC Cancer 24, 776 (2024). https://doi.org/10.1186/s12885-024-12532-y
- CAP TODAY, February 2025. https://www.captodaymag.com/captoday/february_2025/MobilePagedArticle.action?articleId=2042482#articleId2042482; [Accessed April 9, 2025]
- Gargano JW, Stefanos R, Dahl RM, et al. Trends in Cervical Precancers Identified Through Population-Based Surveillance — Human Papillomavirus Vaccine Impact Monitoring Project, Five Sites, United States, 2008–2022. MMWR Morb Mortal Wkly Rep 2025;74:96–101. DOI: http://dx.doi.org/10.15585/mmwr.mm7406a4
- Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. J Low Genit Tract Dis 2020;24:102–31. https://pubmed.ncbi.nlm.nih.gov/32243307/
- Unites States Preventative Services Task Force; https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/cervical-cancer-screening-adults-adolescents; [Accessed April 8. 2025]
- World Health Organization https://iris.who.int/bitstream/handle/10665/342365/9789240030824-eng.pdf?sequence=1 [Accessed April 8. 2025]
- WHO Global strategy to accelerate the elimination of cervical cancer as a public health problem; https://www.who.int/publications/i/item/9789240014107; [Accessed April 8. 2025]
- Young, AP, Olorunfemi, M, Morrison, L. et al. Cervical cancer screening: Impact of collection technique on human papillomavirus detection and genotyping. Preventive Medicine Reports 50 (2025) 102971. https://doi.org/10.1016/j.pmedr.2025.102971
- Wentzensen, N, Massad, LS, Clarke, MA et al. Self-Collected Vaginal Specimens for HPV Testing: Recommendations From the Enduring Consensus Cervical Cancer Screening and Management Guidelines Committee. Journal of Lower Genital Tract Disease, Volume 29, Number 2, April 2025. https://pubmed.ncbi.nlm.nih.gov/39982254/





0 Comments