Adventitious agent testing is critical to ensure patient safety and maintain product integrity. Biopharmaceuticals, often derived from living cells, are susceptible to contamination by viruses and mycoplasmas, which can be introduced during cell culture, raw material handling, or manufacturing processes. Viral contamination can pose serious health risks, including infection or immune responses in patients, while mycoplasmas—tiny bacteria lacking cell walls—can alter cellular functions, compromise product efficacy, and skew quality control results. Rigorous testing helps detect and eliminate these contaminants early, ensuring that the final product is both safe and effective for therapeutic use. This blog post demonstrates the importance of integrating Mycoplasma testing alongside viral safety protocols since Mycoplasma can not only threaten patient health but also degrade cell cultures and compromise testing procedures by altering cell metabolism, genetic expression, and other cellular functions.
Understanding Adventitious Agent Testing (AAT) in Biopharmaceuticals
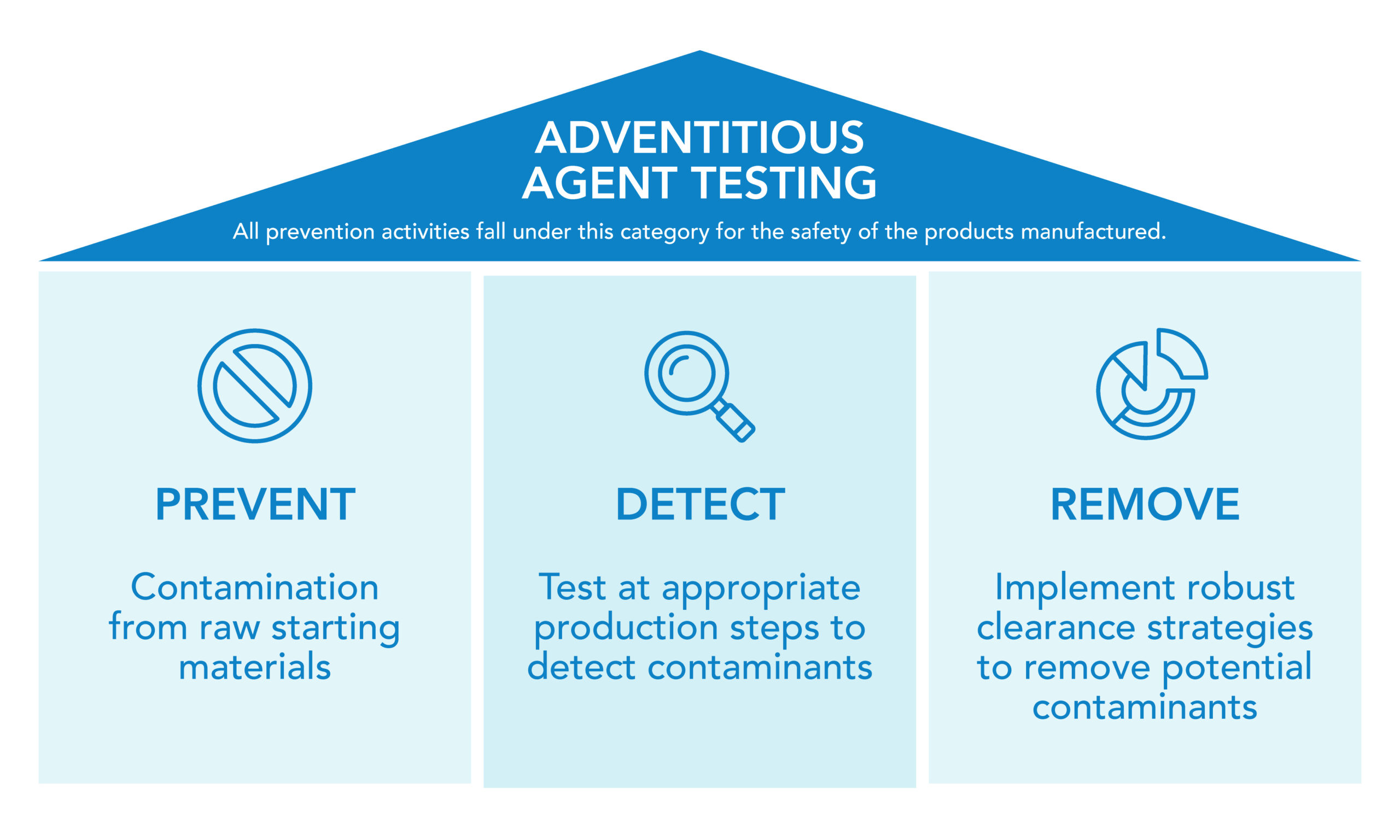
Comprehensive AAT is essential to keep biopharmaceutical products free from viral and microbial contaminants. This level of purity involves a three-pronged approach:
Prevention: Selecting and rigorously testing source materials to minimize contamination risk.
Detection: Conducting regular testing at various stages of production to identify potential threats early.
Removal/Inactivation: Implementing validated manufacturing processes to reduce or eliminate viral contaminants.
Regulatory bodies such as the FDA, EMA, and ICH provide guidelines to ensure the implementation of robust adventitious agent safety measures. Recent advancements, such as next-generation sequencing (NGS), have significantly improved the ability to detect a broader range of viral contaminants. In addition to viral safety measures, the use of cell cultures will typically require Mycoplasma screening to protect patients and the cell lines.
Why Mycoplasma Poses a Multilayered Threat in Biopharmaceutical Manufacturing
The features that make Mycoplasma dangerous to patients also make it difficult to detect and eradicate. Lacking a cell wall, Mycoplasma is resistant to many antibiotics and difficult to detect using several routine methods. Mycoplasma is a common contaminant in cell culture systems, capable of altering cell metabolism, growth, and protein production, ultimately compromising product safety and efficacy.
The Role of Mycoplasma Testing alongside Viral Safety Protocols
To ensure comprehensive safety, Mycoplasma testing should be integrated alongside viral safety protocols for any products that rely on cell lines. Both types of testing can be conducted at key points in the production process, including raw material testing, in-process monitoring, and final product verification.
Mycoplasma’s Fit Within the Three Pillars of Adventitious Agent Testing
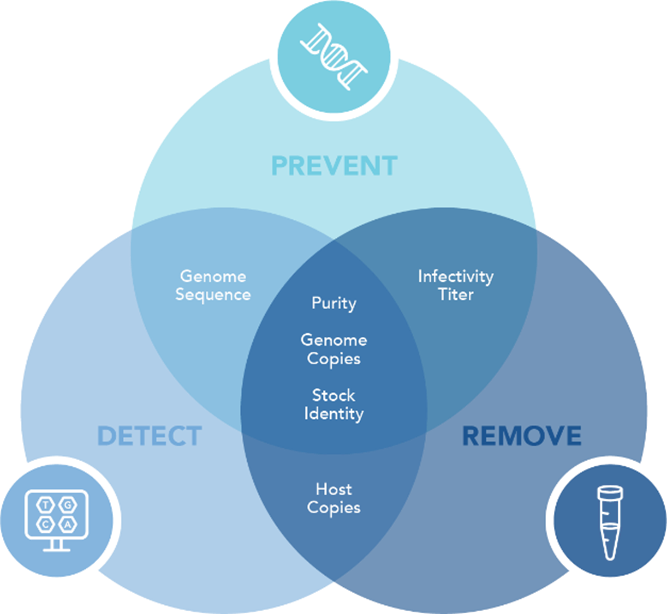
Detect
Mycoplasma contamination can be detected through NGS and PCR-based methods, ensuring contamination control in cell cultures and biopharmaceutical production.
Prevent
Ensuring mycoplasma-free cultures is critical for maintaining viral stock integrity, preventing misinterpretation of safety studies, and ensuring product consistency. Effective prevention of mycoplasma contamination is essential to uphold these standards.
Remove
While not a virus, Mycoplasma can alter host cell metabolism, impacting viral growth and infectivity studies, potentially leading to false results in viral titration assays.
By integrating Mycoplasma detection alongside viral safety protocols, biopharmaceutical manufacturers enhance their ability to maintain contamination-free production environments.
Regulatory Requirements for Mycoplasma and Viral Testing
Regulatory agencies worldwide mandate stringent testing protocols to mitigate both viral and mycoplasma contamination risks. Guidelines from organizations such as the FDA and ICH emphasize:
1. Validated Testing Methodologies
Regulatory agencies like the FDA, EMA, and WHO require validated testing methodologies to ensure the accuracy and reliability of viral safety and mycoplasma testing. These methodologies must be validated according to Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards. The validation process includes demonstrating the method’s sensitivity, specificity, accuracy, precision, and robustness. 1, 2
2. Routine Monitoring at Critical Stages of Production
Routine monitoring is mandated at critical stages of production to ensure the absence of viral and mycoplasma contamination. This includes regular testing of raw materials, in-process samples, and final products. The United States Pharmacopoeia (USP), European Pharmacopoeia (EP), and other regulatory bodies provide guidelines for the frequency and methods of monitoring.2, 3
3. Advanced Detection Technologies
Advanced detection technologies, such as molecular-based assays like Polymerase Chain Reaction (PCR) and Next-Generation Sequencing (NGS), are increasingly being adopted for their high sensitivity and specificity. Regulatory guidelines, including ICH Q5A(R2), emphasize the use of these technologies for rapid and sensitive detection of adventitious and endogenous viruses.4, 5 These assays must also be validated according to regulatory standards to ensure their reliability and accuracy.5
Mycoplasma Testing Methods and Their Application
Several mycoplasma detection methods exist, each with its own strengths and limitations:
Culture Methods: Highly specific but time-consuming (ranging from 48 hours to four weeks).
Nucleic Acid Amplification Techniques (NAAT): Rapid and sensitive, making them ideal for routine monitoring.
Indicator Cell Culture: Less commonly used due to its complexity and longer turnaround time.
Choosing the Right Mycoplasma Controls: Genomic vs. Inactivated
The selection of appropriate mycoplasma controls depends on the testing methodology and stage of production:
Genomic Controls: Ideal for molecular-based assays like PCR and qPCR, supporting sensitivity testing, method validation, and routine monitoring.
Inactivated Controls: Suitable for culture-based methods, process validation, and training, mimicking real contamination conditions without the risk of live bacteria.
In-House vs. Ready-to-Use Mycoplasma Controls
Biopharmaceutical manufacturers have the option to either produce Mycoplasma controls in-house or purchase ready-to-use reference materials from suppliers like Microbiologics. Each approach has its own benefits and challenges:
In-House Production: Allows for customization but requires significant time, resources, and expertise.
Ready-to-Use Reference Materials: Ensure consistent quality and regulatory compliance while reducing time and labor requirements. Microbiologics provides high-quality, validated mycoplasma and viral safety reference materials, supporting manufacturers with reliable solutions for contamination control.
The Importance of Comprehensive QC Testing
By integrating Mycoplasma testing within viral safety protocols, manufacturers can achieve:
Enhanced Product Safety: Ensuring products are free from both viral and mycoplasma contaminants.
Regulatory Compliance: Meeting global standards to avoid recalls and ensure smooth approvals.
Improved Product Quality: Reliable testing methods contribute to overall product consistency and efficacy.
The Comprehensive Safety Program Patients Deserve
As the biopharmaceutical industry advances, robust QC processes remain essential to ensuring patient safety. Incorporating mycoplasma testing along with viral safety protocols will strengthen contamination control measures. By adopting advanced testing technologies and adhering to regulatory guidelines, manufacturers can safeguard their products and maintain industry-leading quality standards.
A Proven Partner in Viral and Mycoplasma Safety
Microbiologics remains committed to supporting the industry with innovative solutions that ensure the highest levels of safety and compliance. As a leader in the biopharmaceutical quality control space, Microbiologics offers comprehensive solutions to support both viral safety and mycoplasma testing. Our extensive portfolio includes:
Ready-to-use mycoplasma controls for molecular and culture-based detection.
NGS-compatible viral safety reference materials to enhance contaminant detection capabilities.
Custom QC solutions tailored to meet the evolving needs of biopharmaceutical manufacturers.
By leveraging our expertise and high-quality controls, manufacturers can confidently enhance their viral and mycoplasma safety programs, ensuring compliance with global regulations and safeguarding product integrity. Contact us to set up a QC consultation for your adventitious agent screening program.
References
1. Weber, S. M., et al. Mycoplasma testing of biopharmaceuticals and ATMP: Current regulations, challenges and trends. Roche. Dec. 2021. https://custombiotech.roche.com/content/dam/acadia/applicationnotes/560/42/booklet%20MycoTOOL.pdf.
2. Kalaivani, M. “Current Regulatory and Pharmacopoeial Status of Mycoplasma Testing in Concern with Vaccines Safety for Human Use.” Indian Pharmacopoeia Commission. Jun. 2021. https://www.jbclinpharm.org/articles/current-regulatory-and-pharmacopoeial-status-of-mycoplasma-testing-in-concern-with-vaccines-safety-for-human-use-8485.html.
3. Marians, R. C. “Regulatory Aspects of Mycoplasma Testing for Cell Therapy Products.” Labs, Inc. Jun. 2014. http://stem-art.com/Library/Miscellaneous/Regulatory%20Aspects%20of%20Mycoplasma%20Testing%20for%20Cell%20Therapy%20Products.pdf.
4. Khan, A. S. “ICH Q5A(R2) Updates: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin.” FDA. Jul. 2021. https://www.casss.org/docs/default-source/cmc-strategy-forum-north-america/2021-cmc-na-speaker-presentations/khan-arifa-cber-fda-2021.pdf.
5. “Clinical Testing & Diagnostics Manufacturing.” Millipore Sigma. https://www.sigmaaldrich.com/US/en/applications/clinical-testing-and-diagnostics-manufacturing.





0 Comments