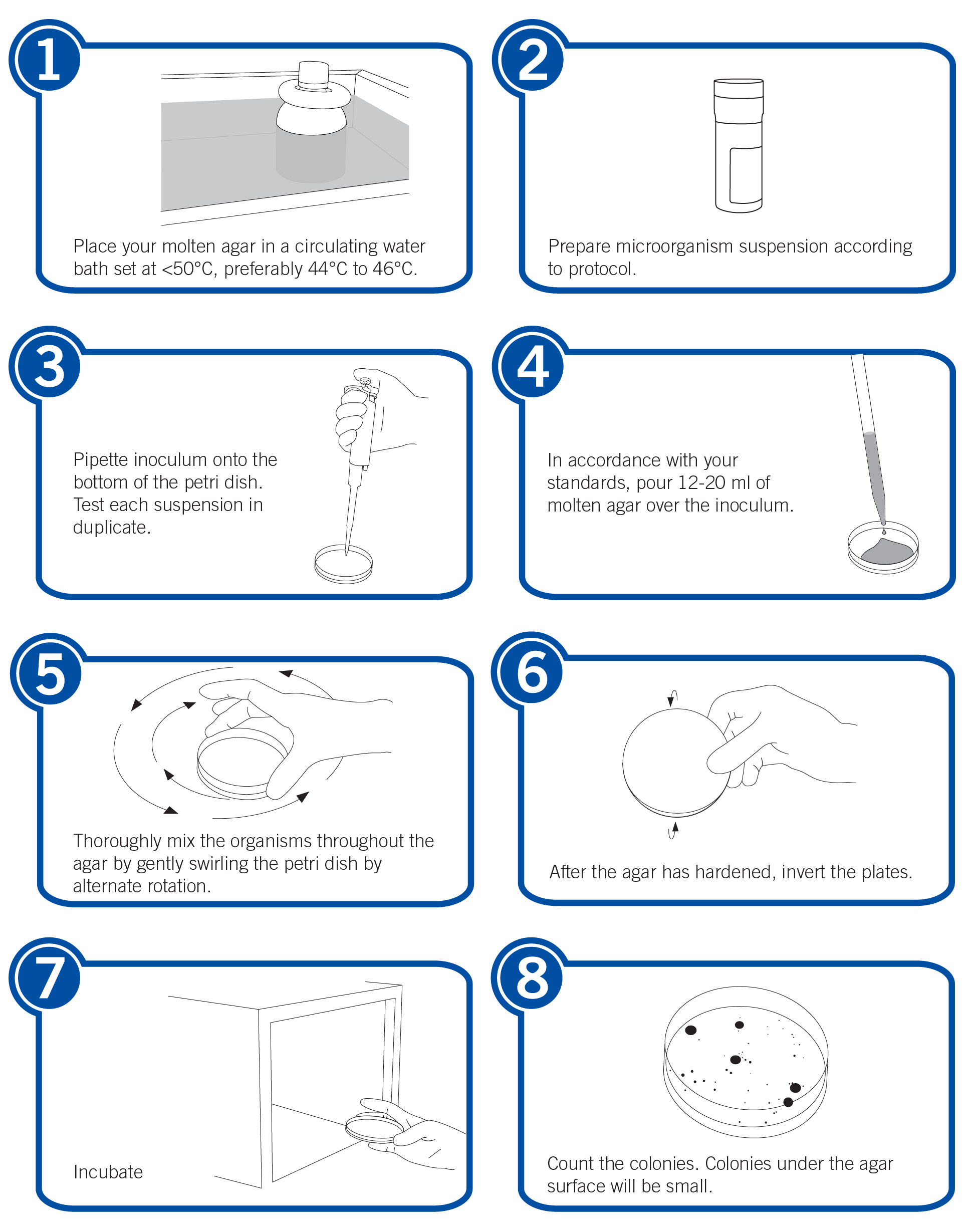
The pour plate method is an economical way for pharmaceutical, contract and even food laboratories to perform tests focused on a specific number of bacteria. The process may seem simple (melt, pipette, pour, swirl, incubate), but errors have been known to occur.
Fortunately, there are several steps you can take to ensure the success of your test. Try implementing these 11 best practices the next time you use the pour plate method for growth promotion testing (GPT), microbial enumeration, or bioburden testing.
1. Keep the molten agar in the water bath for no more than three to four hours. Don’t pour the agar until it has cooled to <50°C (preferably 44°C to 46°C).
2. Don’t re-melt the agar. Agar should only be melted one time.
3. Use phosphate buffer pH 7.2 if necessary to dilute the suspension.
4. Decrease the risk of contamination by pouring plates in a laminar-airflow cabinet. When pouring multiple plates, flame the mouth of the flask before moving on to the next plate to reduce the risk of contamination.
5. Fill plates according regulators’ recommendations. The U.S. Food and Drug Administration (FDA) Bacteriological Analytical Manual (BAM) recommends filling plates with 12 ml to 15 ml of agar. The United States Pharmacopeia (USP) recommends a fill of 15 ml to 20 ml of agar.
6. Some microorganism species, such as obligate aerobes, may recover better on spread plates than pour plates. When growing these strains, it is recommended to verify counts with a spread plate.
7. Incubate most bacterial species for 48 to 72 hours. Note: Incubate Candida albicans and Aspergillus brasiliensis for three to five days.
8. Count small microorganism colonies, such as Pseudomonas aeruginosa, with the aid of an illuminated colony counter or magnifying glass.
9. Use the formula below to determine the number of CFU per ml:
![]()
10. Keep in mind recovery will be lower on selective agar. If selective agar is used, test non-selective agar in parallel using the same microorganism suspension. A higher CFU concentration for the selective agar may be necessary.
11. Don’t be surprised if the value obtained when performing tests differs from the mean assay value. Note: Microbiologics uses non-selective Tryptic Soy Agar when testing most microorganisms.
Download a PDF of our pour plate best practices and instructions.
Here are the steps we follow in our laboratory to successfully perform the pour plate method:
Visit our website for technical documents, illustrated instructions, instructional videos, FAQs and more.
References
International Standard for Organization. 2009-02-01. Second Edition: Microbiology of food and animal feeding stuffs – Guidelines on preparations and production of culture media. Part 1
Maturin, L. and Peeler, J. T. 2001. FDA Bacteriological Analytical Manual. Chapter 3, Aerobic Plate Count.
Standard Methods for the Examination of Dairy Products. 2004. 17th Edition
Standard Methods for the Examination of Water and Wastewater. 2005. 9020, Quality Assurance/Quality Control.
United States Pharmacopeia. 2013. <61>, Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests.





Or automate production with one of our machines. They also reduce almost all human errors and give a steady production speed.
Good traceability and far more consistency.
Awesome information….thank you
Thank you for your recommendation, I will share it with my team
Thanks a lot.
Good article to read. What should be the pour volume for plates used in Environmental Monitoring?
Deepak – We recommend contacting your regulatory agency for the appropriate pour volume of agar for environmental monitoring plates.
Hello, I have a question about the composition of the medium, is there eny recommendation about amount of containing agar? Thank you.
Hi Anja, thank you for your interest! For clarification, are you referring to the amount of agar added to the media itself or the volume of media poured into the Petri dish?
Hello,I want to know what would cause a colony not to grow when using the spread plate.
Hi Vanessa,
Thank you for reaching out! There are many factors that could cause a culture not to grow. Some of the factors could be that the media is not able to support growth (this is why performing growth promotion is essential), the incorrect media or incubation conditions were used, or the loop/spreader was too hot prior to spreading the suspension around. To rule out some of these options, you should perform growth promotion on every batch of media, use a disposable, sterile spreader, and monitor incubator conditions. If you have further questions, please email us at techsupport@microbiologics.com .