When summer is in full swing, many of us choose to cool down with sweet frozen dairy treats. However, the thought of ice cream tainted with Listeria monocytogenes may cause anyone, especially those in susceptible populations, to think twice.
Listeria monocytogenes is a pathogenic bacteria species that naturally occurs in the environment. This pathogen can contaminate food and cause mild to severe illnesses, sometimes resulting in death. There are certain foods that are viewed by the public as more susceptible to L. monocytogenes contamination, but it could potentially be found in almost any ready-to-eat (RTE) food. Check out our Environmental Isolate Case Files: Listeria monocytogenes to learn more about this notorious strain.
The presence of Listeria species in frozen ice cream products has been linked to numerous illnesses and even deaths. A highly visible case was the FDA recall involving Texas-based Blue Bell ice cream, with a L. monocytogenes outbreak spanning 4 states. In the end, 10 illnesses were reported to the CDC1 resulting in 3 deaths. The company’s 3 facilities that produced product with positive results for L. monocytogenes were examined for potential sources of contamination. Ultimately, it was discovered that the bacteria were introduced to each facility through multiple routes and a single mode of entry could not be established. After introduction of L. monocytogenes to these manufacturing plants, it proceeded to colonize the facility and take root where it could then be transferred to the ice cream products. A Texas manufacturing plant harbored2 L. monocytogenes in pieces of equipment including some in the production line, while a plant in Oklahoma was found3 to have an infested drain system. In the companies’ root cause analysis of the outbreak at each location, steps taken to eliminate and prevent future contamination were outlined.
Those steps resemble the FDA’s draft guidance for industry in the control of L. monocytogenes in RTE foods, where a routine of decontamination and testing is recommended to be followed by all manufacturers of this food category.
Ice cream was not always officially considered by the FDA4 to be an RTE food that supports growth of L. monocytogenes. That did not mean that ice cream could not contain the bacteria, but rather that since the conditions in which it was produced and stored meant that the food carried a low-risk for contamination. Frozen foods were not then considered to be high risk since L. monocytogenes has inhibited growth below 0º C (32º F). Even though growth may be reduced at or below this temperature threshold, L. monocytogenes can still slowly multiply in refrigerated foods. Frozen foods can also become contaminated through food contact surfaces, tainted ingredients, and introduction of pathogens by employees.
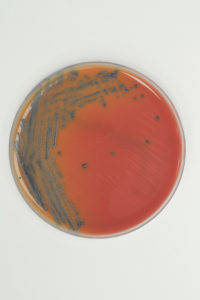
Listeria monocytogenes (Microbiologics Cat. No. 0129P) grown on RAPID’L.mono Agar. Colonies are blue with no halo after 24-48 hours of incubation at 37ºC.
In its latest update to their Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-to-Eat Foods, the FDA states5 that only the following conditions can inhibit growth of L. monocytogenes:
- when food pH is equal to or less than 4.4 (e.g., foods with high vinegar content)
- when the food’s water activity is less than or equal to 0.92
- when the food can be scientifically proven to inhibit growth through a variety of combined antibacterial factors
Basically, if a RTE food carries the potential to be eaten raw or in its unadulterated state, even if it is frozen, then it would probably fall under this draft guidance update. This means a significant portion of manufacturing locations now fall under the RTE guidelines where they may not have before. These new guidelines and recommendations are designed to eliminate L. monocytogenes’ introduction to facilities by personnel, equipment, and supplies, as well as continuously monitor facilities for presence of the pathogen.
Knowing this, it is now up to frozen food manufacturers to adhere to the new regulations in their RTE manufacturing facilities. For many this means implementation of a new environmental monitoring program and increased sanitization procedures aimed at eliminating cross-contamination of RTE food.
A critical part of maintaining a manufacturing facility free of L. monocytogenes is a strong environmental monitoring program. According to the FDA6, the objectives of that program should accomplish the following:
- Proof that implemented listericidal control programs work as expected
- Are your facility’s sterilization procedures stringent enough?
- Discovery of monocytogenes contamination if present in the facility, not just the final product
- Promotion of thorough environmental sampling, both on surfaces contacted by food and surfaces and areas that do not, will discover lurking contamination
- Corrective action procedures
- If and when Listeria contamination is present in the facility, what actions will be taken?
Adapting to these updated regulations may cause quite the (ice cream) headache for RTE food manufacturers, but when the cost of improper practices can mean death to consumers, following them is in everyone’s best interests.
Fortunately, Microbiologics offers a host of products that can fit into a new or existing program used as environmental monitoring controls:
The FDA recommends the following controls in BAM (Bacteriological Analytical Manual):
| Microorganism Strain | Comment |
| Listeria monocytogenes derived from ATCC® 19115™* | Serotype 4b |
| Listeria innocua derived from ATCC® 33090™* | Serotype 6a |
| Listeria seeligeri derived from ATCC® 35967™* | |
| Listeria ivanovii subsp. ivanovii derived from ATCC® 19119™* | |
| Rhodococcus equi derived from ATCC® 6939™* | Used for CAMP Test |
| Staphylococcus aureus subsp. aureus derived from ATCC® 25923™* | Used for CAMP Test |
ISO 11133 recommends the following controls to test Agar Listeria:
| Control | WDCM # / (Microbiologics Cat No.) | CFU | Reaction |
| L. monocytogenes | 00021 (0129)
00109* (01190) |
80-120 | Blue green colonies with opaque halo |
| E. coli
E. faecalis |
00012 (0483) or 00013 (0335) | 103 to 104 | No growth |
| L. inocua | 00017 (0414 or 0814) | 104 to 106 | Blue green colonies without opaque halo |
*Optional
References
- S. Centers for Disease Control and Prevention. Multistate Outbreak of Listeriosis Linked to Blue Bell Creameries Products (Final Update). (June 10, 2015). Retrieved from https://www.cdc.gov/listeria/outbreaks/ice-cream-03-15/index.html
- S. Food and Drug Administration. Blue Bell Creameries Inc. Summary of Root Cause Assessment Brenham, Texas, Facility. (February 12, 2016). Retrieved from https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm492436.pdf
- S. Food and Drug Administration. Blue Bell Creameries Inc. Summary of Root Cause Assessment Broken Arrow, Oklahoma, Facility. (February 12, 2016). Retrieved from https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm492435.pdf
- S. Food and Drug Administration. CPG Sec. 555.320 Listeria monocytogenes. (February 2008). Retrieved from https://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm136694.htm
- S. Food and Drug Administration. Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-To-Eat Foods. (January 13, 2017) Page 7. Retrieved from https://www.fda.gov/RegulatoryInformation/Guidances/ucm073110.htm
- S. Food and Drug Administration. Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-To-Eat Foods. (January 13, 2017) Page 39. Retrieved from https://www.fda.gov/RegulatoryInformation/Guidances/ucm073110.htm





0 Comments