They outnumber your human cells. They influence everything from digestion to immunity. And now, microbes are reshaping how we understand and treat disease. The microbiome, the vast community of microorganisms living in and on our bodies, plays a crucial role in maintaining health and preventing illness.
The Microbiome’s Role in Human Health
Microbes reside in all parts of the human body, contributing to and influencing digestion, immunity, and aging. The skin, nasal, and oral microbiota are an important part of our first line of defense against pathogens and help strengthen the immune system. On the skin, they prevent disease by filling available nutritional niches which competitively excludes pathogens from colonizing the skin.1 Skin microbes also continuously secrete microbial factors which help calibrate the immune system so that it remains ready to fight off non-resident pathogenic microbes.1 Resident microbes in the nose and mouth influence production of mucus and antimicrobial chemicals, and oral microbes assist in the first stages of digestion.2,3 The ability for resident microbes to stimulate mucus production also helps maintain lubrication and mucosa-associated host defenses critical to proper lung function.4 Complex and diverse communities of microbes in the stomach help regulate gastric functions and play a major role in digesting complex carbohydrates in the colon.5 Organisms associated with the urogenital tract help exclude other microbes, and play a critical role in maintaining a healthy vaginal pH.6 As researchers learn more about the role of microbes in our health, new paths for disease prevention and therapy have emerged.
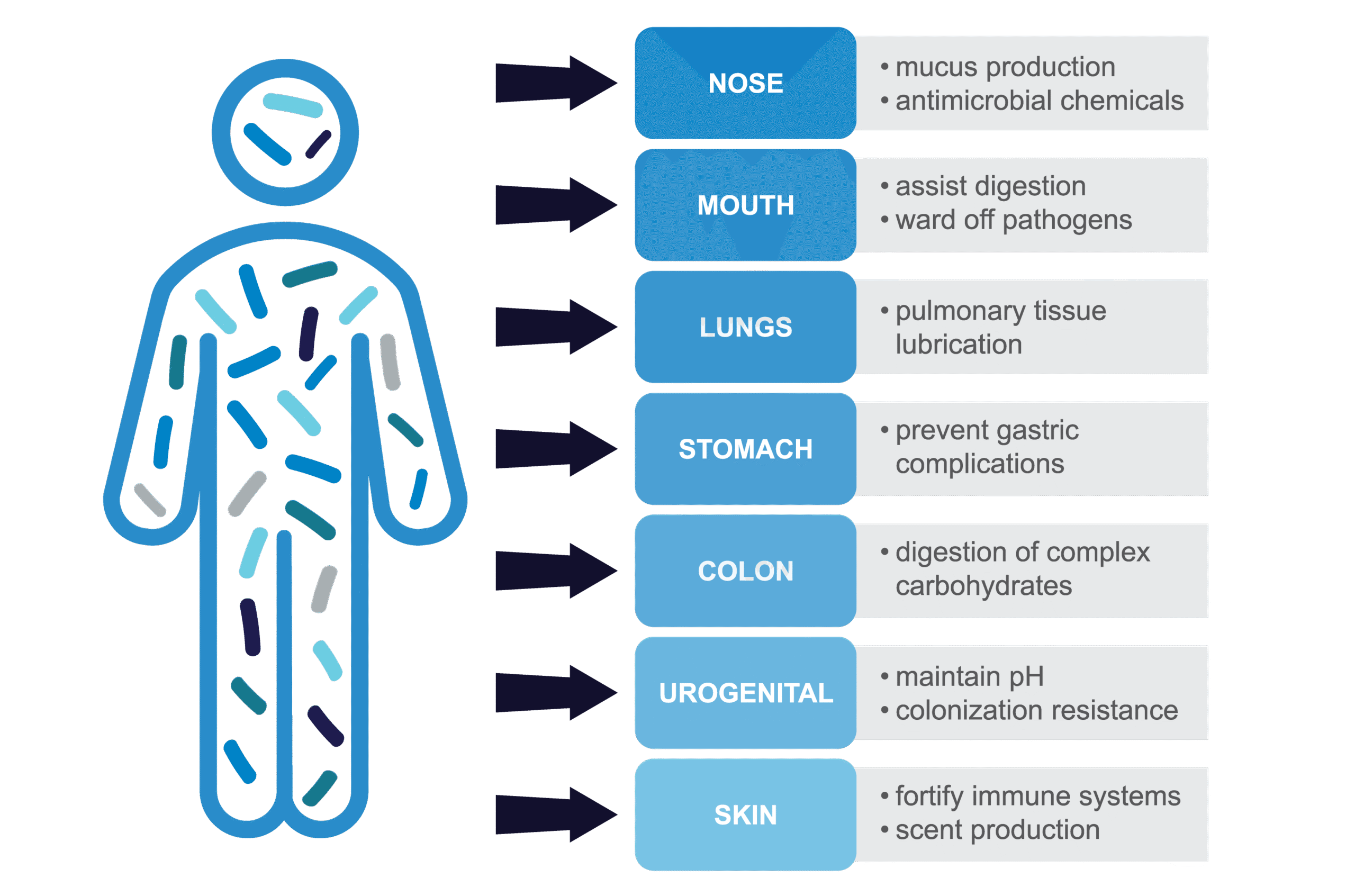
Figure 1. The many roles of microbiota in the human body. Figure adapted from Appana 2018.15
Insights around the role of microbes in human health has led to groundbreaking translational research that has the potential to reshape the future of medicine. One of the biggest hurdles in developing products to repair or reshape the human microbiome is its complexity and the feasibility of translating pre-clinical findings into clinical applications.7 Each person has a unique mix of microbes influenced by factors like genetics, diet, and lifestyle adding to the complexity of targeting and tracking therapeutic outcomes.8
From Discovery to Translational Research
The first in-depth characterization of the human microbiome was completed as a part of the first phase of the Human Microbiome Project using healthy volunteers, and laid the foundation for further research.9 Phase 2 of the Human Microbiome Project provided deeper characterizations of the human microbiome that explored a broader set of biological properties and tracked cohorts of participants with microbiome-associated conditions (pregnancy and preterm birth, inflammatory bowel disease, prediabetes) over time.9 The rigorous multi-omics methods developed and applied during these two phases of the Human Microbiome Project continue to shape how microbiome research is conducted.9
Live Biotherapeutic Products
As researchers continue to uncover the impact that microbes have on human health, entrepreneurs have begun turning these discoveries into clinical treatments by developing microbiome-based live biotherapeutic products (LBPs). LBPs can be broadly categorized based on the level of detail known about their composition (undefined or defined). LBPs with undefined composition include products using material derived from healthy donors that have been screened and processed to lessen the potential for transfer of disease from donors to the therapy recipient. To date, two donor-derived LBPs are the only products that have received FDA-approval.10 Defined composition LBPs composed of either single strains or multi-strain consortia derived from pure cultures are still in development.11
A goal for many microbiome products being developed is to restore a patient’s microbiome after they receive antimicrobial therapy. In this context, it is important to understand the susceptibility of strains in an LBP to antibiotics, as well as the potential for the LBP to convey resistance determinants via horizontal gene transfer.12 By way of example, the two FDA-approved LBPs were developed for patients with recurrent Clostridioides difficile infections that repeatedly failed treatment with antimicrobials.10 This indication has been a primary interest in LBP development because C. difficile infections account for half a million infections and 30,000 deaths annually.13 Moreover, around 60% of cases begin within 4 months of antimicrobial therapy, and 20 to 35% of patients experience at least one recurrence.13 In contrast to use of broad-spectrum antimicrobials, use of an LBP in concert with or instead of antimicrobials has the capacity to maintain microbiome diversity, a critical component of resistance to colonization by pathogens, including those that may harbor antimicrobial resistance.14
Navigating Regulation and Product Development
FDA approval is required to market an LBP with a specific health claim. Just like pharmaceutical drugs or medical devices, LBPs must undergo rigorous testing prior to and during clinical trials.12 As one example, knowledge of a strain’s susceptibility to antibiotics is important in the context of antimicrobial stewardship and as a regulatory consideration. The Guidance for Industry on Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information published by the FDA in 2016 includes guidance to determine the minimum inhibitory concentrations of relevant antibiotics against strains used in an LBP.12 Product developers may also need to consider how bacteria in their product interact, the stability of the product overtime, and how probiotics/diet may influence the effectiveness for the targeted therapeutic application.14 Taking together the FDA guidance and other product development considerations, LBP developers should consider the following when characterizing and developing their LBP:
- Characterize the strains genetically and biochemically
- Confirm the proportions and abundance of bacteria
- Evaluate the effects of both probiotics and prebiotics
- Test the stability of the product over time and in different environments
- Evaluate the susceptibility of strains to antimicrobials with antibiograms
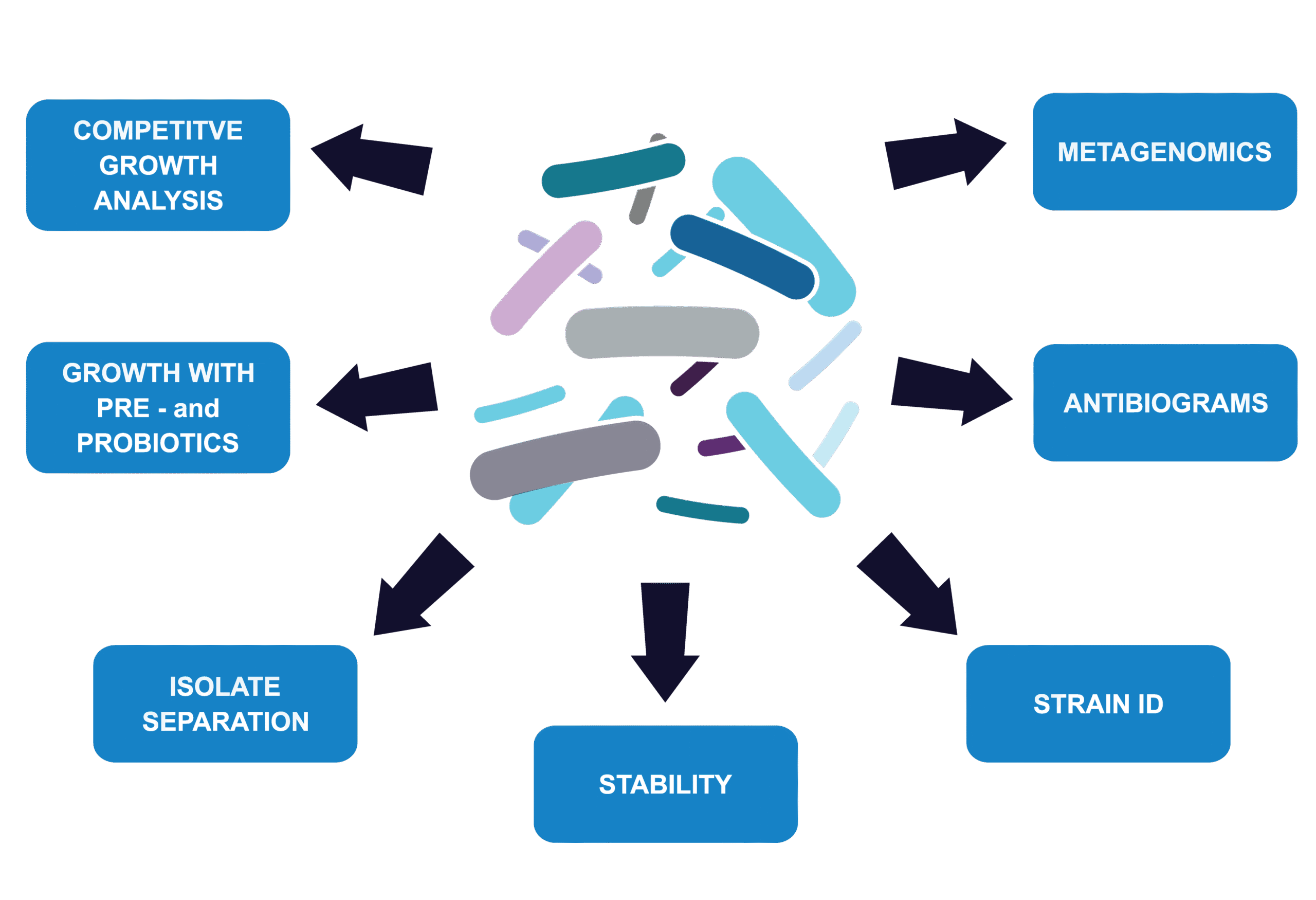
Figure 2. Summary of services offered by Microbiologics to characterize your LBP or mixed bacterial sample.
How Microbiologics Can Partner with Research
If you are an innovator developing products for microbiome-based therapy, Microbiologics offers advanced capabilities to support your program at every stage. Our team can assist with the in-depth characterization of LBPs or mixed bacterial samples (Figure 2), applying proven assays and analytical methods to generate data you can trust. When it’s time to prepare your IND, we also provide scientific writing and regulatory consulting services designed to help streamline submissions and accelerate approval pathways.
Special thanks to Andrea Marra, PhD, Business Development Manager at Microbiologics, for her contributions to this article and her collaboration with teams bringing microbiome innovations from lab to clinic.
References
- Chen YE, Fischbach MA, Belkaid Y. Skin microbiota–host interactions. Nature. 2018;553(7689):427-436. doi:10.1038/nature25177
- Deo PN, Deshmukh R. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122-128. doi:10.4103/jomfp.JOMFP_304_18
- Salzano FA, Marino L, Salzano G, et al. Microbiota Composition and the Integration of Exogenous and Endogenous Signals in Reactive Nasal Inflammation. J Immunol Res. 2018;2018:2724951. doi:10.1155/2018/2724951
- Dickson RP, Huffnagle GB. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015;11(7):e1004923. doi:10.1371/journal.ppat.1004923
- Sanz Y, Cryan JF, Deschasaux-Tanguy M, Elinav E, Lambrecht R, Veiga P. The gut microbiome connects nutrition and human health. Nat Rev Gastroenterol Hepatol. 2025;22(8):534-555. doi:10.1038/s41575-025-01077-5
- France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol. 2022;7(3):367-378. doi:10.1038/s41564-022-01083-2
- Metwaly A, Kriaa A, Hassani Z, et al. A Consensus Statement on establishing causality, therapeutic applications and the use of preclinical models in microbiome research. Nat Rev Gastroenterol Hepatol. 2025;22(5):343-356. doi:10.1038/s41575-025-01041-3
- Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565-569. doi:10.1126/science.aad3369
- Proctor LM, Creasy HH, Fettweis JM, et al. The Integrative Human Microbiome Project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- FDA (Food and Drug Administration). Fecal Microbiota Products. FDA. June 7, 2024. Accessed July 17, 2025. https://www.fda.gov/vaccines-blood-biologics/fecal-microbiota-products
- Gilbert JA, Azad MB, Bäckhed F, et al. Clinical translation of microbiome research. Nat Med. 2025;31(4):1099-1113. doi:10.1038/s41591-025-03615-9
- FDA. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information; Guidance for Industry.
- Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infectious Diseases. 2023;23(1):132. doi:10.1186/s12879-023-08096-0
- Ducarmon QR, Kuijper EJ, Olle B. Opportunities and Challenges in Development of Live Biotherapeutic Products to Fight Infections. The Journal of Infectious Diseases. 2021;223(Supplement_3):S283-S289. doi:10.1093/infdis/jiaa779
- Appanna VD. 2018. The Microbiome: Genesis and Functions, p. 37–79. Human Microbes – The Power Within. Springer, Singapore.





0 Comments