Pluralibacter gergoviae can cause big headaches in cosmetic industry laboratories. It is an opportunistic pathogen that has repeatedly been isolated from personal care products. Most recently, this environmental isolate is the cause of a recall of involving flushable wipes. (6)
Never heard of the species? You may be familiar with its former name, Enterobacter gergoviae. The species received a new name based on multilocus sequence analysis. (3,9)
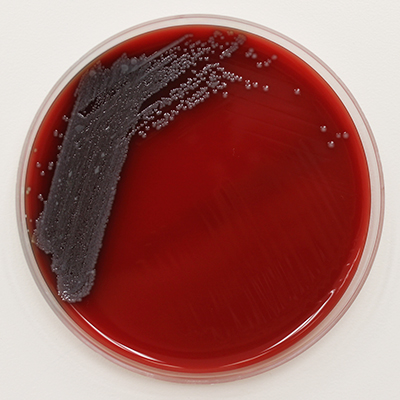
Pluralibacter gergoviae on Blood Agar
Description:
Pluralibacter gergoviae is a Gram-negative rod. It is facultatively anaerobic and motile by peritrichous flagella. Colonies are unpigmented, round and smooth on Tryptic Soy Agar. It grows optimally at 30°C. (3)
Environmental Sources:
The species has been isolated from soil, water, cosmetics, sewage, maize, grape bay, coffee beans, refrigerated packed fish paste, brown leaf spots on pear trees, and the insect guts of the fruit fly and the pink bollworm. (3,7,10)
Pathogenicity:
Pluralibacter gergoviae has been recovered from the human respiratory tract, urinary tract and blood. (1) It was reported to be the cause of outbreaks of nosocomial bacteremias in Croatia neonatal intensive care units. (2) The species is often resistant to antibiotics, making treatment more difficult. (8)
Contaminant:
The strain has been isolated from several personal care products including exfoliating gel, eyeliner, foundation cream, shampoo, rose bath, shower gel, shea butter, conditioner, bubble bath and baby wipes. (8,10,11) Pluralibacter gergoviae has also been isolated from the floors of the washing area of an industrial plant. (5)
FDA Warning Letter:
The FDA issued a warning letter in 2016 to a manufacturer of shower gel, shampoo and conditioner after it found an excessive level of microorganisms in the products. One of the species recovered was Pluralibacter gergoviae (formerly E. gergoviae). In the warning letter, the FDA said, “E. gergoviae are part of the normal intestinal flora and may be considered an opportunistic pathogen. Individuals with weakened immune systems, who suffer from a serious pre-existing condition, who have been treated surgically or belong to another sensitive group of persons are at particular risk of infection.” (4)
FDA criticized the company because:
- It did not perform any microbial testing on any incoming raw materials or validate the suppliers’ quality testing through independent verification.
- It did not maintain the equipment used to manufacture the products in a clean and orderly condition or sanitize it at appropriate times
The FDA recommended that the company implement quality controls and/or reconditioning processes to ensure the safety of the products it manufactures. It also recommended following the FDA Good Manufacturing Practice Guidelines/Inspection Checklist for Cosmetics. (4)
Resistance:
Pluralibacter gergoviae has an innate resistance to parabens such as methylparaben and propylparaben due to production of an Enzyme PrbA and an efflux mechanism. (12)
Family:
- Phylum: Proteobacteria
- Class: Gammaproteobacteria
- Order: Enterobacteriales
- Family: Enterobacteriaceae
- Genus: Pluralibacter
Environmental Isolate Controls:
Cosmetic and personal care product manufacturers are increasingly seeking to incorporate environmental isolates, like Pluralibacter gergoviae, into their routine laboratory Quality Control (QC) processes to ensure product quality and meet regulatory standards. Microbiologics offers a comprehensive program for environmental isolate management. Our team of experts will identify, characterize, preserve and manufacture your strain into a test-ready control format for ongoing QC. Check out our Custom Solutions offering to learn more and get connected with our team of experts.
References:
(1) Abbott, S. L. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and Other Enterobacteriaceae. Manual of Clinical Microbiology. 10th Volume 1. P. 639-657.
(2) Boban, N., Jeroničić, A., Puda-Polić, V. Outbreak of nosocomial bacteremia, caused by Enterobacter gergoviae and Enterobacter aerogenes, in the neonatal intensive care unit, case-control study. Sigma Vitae 2011; 6(1): 27-32.
(3) Brady, C., Cleenwerck, I., Venter, S., Coutinho, T., De Vos, P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA). Systematic and Applied Microbiology. 36 (2013) 309-319.
(4) FDA Warning Letters, 2016. Inspections, Compliance, Enforcement, and Criminal Investigations.
(5) Ferrarese, L. Paglia, R., Ghirardini, A. Bacterial resistance in cosmetics industrial plant: connected problems and their solution. (2003) Annals of Microbiology, 53 (4), 477-490.
(6) Kimberly Clark, Cottonelle, Product Recall. 10/27/2020.
(7) Kok-Gan Chana, Kok Keng Teeb, Wai-Fong Yina, Jia-Yi Tana. Complete Genome Sequence of Pluralibacter gergoviae FB2, an N-Acyl Homoserine Lactone-Degrading Strain Isolated from Packed Fish Paste. American Society for Microbiology. Genome Announcements. November/December 2014 2no. 6 e01276-14.
(8) Neza, E. Centini, M. Microbiologically Contaminated and Over-Preserved Cosmetic Products According Rapex 2008–2014. MDPI. Cosmetics 2016, 3, 3.
(9) Oren, A. and Garrity, G. List of new names and new combinations previously effectively, but not validly, published. International Journal of Systematic and Evolutionary Microbiology (2013), 63, 3931–3934.
(10) Périamé, M., Pagés, J.M. Enterobacter gergoviae adaptation to preservatives commonly used in cosmetics industry. International journal of cosmetic science. May 2014.
(11) Sutton, S., Jimenez, L. A Review of Reported Recalls Involving Microbiological Control 2004-2011 with Emphasis on FDA Considerations of “Objectionable Organisms”. American Pharmaceutical Review. January 2012.
(12) Valkova, N., Lépine, F., Bollet, C., Dupont, M., Villemur, R. prbA, a Gene Coding for an Esterase Hydrolyzing Parabens in Enterobacter cloacae and Enterobacter gergoviae Strains. J. Bacteriol. 2002 Sep; 184(18); 5011-5017.





0 Comments