Dear Stanley,
When performing growth promotion with Microbiologics products, do you recommend testing in parallel with a previously approved media? The USP, EP, and JP don’t require testing in parallel. They only state “Test each batch of ready-prepared medium and each batch of medium prepared either from dehydrated medium or from the ingredients described.”
Sincerely,
Joe from Newark, NJ
Dear Joe,
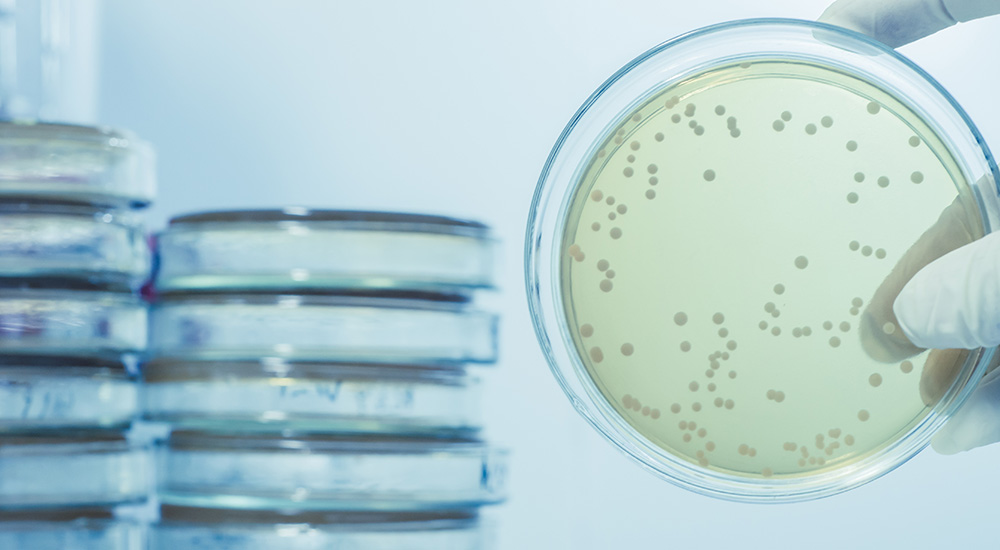
This is a great question. It’s true that the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Japanese Pharmacopoeia (JP) don’t require testing in parallel with a previously approved media when performing the growth promotion test (GPT). However, you may find that testing in parallel helps you to cut down on investigation time if you happen to have a GPT failure.
Testing in parallel eliminates these variables:
• Technician technique: The suspension will be prepared by one technician using the his or her individual pipetting and spreading techniques.
• Incubator temperature: All plates will be exposed to the same temperature and fluctuations in temperature throughout the incubation time.
• Time in incubator: All plates are incubated for the same amount of time.
• Microorganism suspension: The suspension is prepared by one technician from the same hydration fluid and pellet(s).
So, if you test in parallel, the media is the only remaining variable! Here’s how it works. The average count from the previously approved lot is used to calculate the factor of two. The average count on the new lot must fit within this factor of two to pass the lot for growth promotion. This means the value you are comparing to was determined using the same equipment, technique and conditions in your lab at the time growth promotion is performed, giving a true measure of the new lots growth promoting capabilities.
If you don’t have the option to test in parallel because no previously approved media remains, you can use past growth promotion data to calculate the factor of two. Keep in mind, using past data will reintroduce several variables: technician technique, incubator temperature, time in incubator and preparation of the microorganism suspension. If you choose this route, always use the same lot of microorganism suspension used to obtain the previous data.
For more GPT tips, check out our 8 Best Practices for Growth Promotion Testing. If you’d like one-on-one guidance, our Technical Support team is available to answer any questions you have about our products. You can reach them at techsupport@microbiologics.com or 1.320.229.7045
Sincerely,
Stanley Staphylococcus
Stanley Staphylo coccus is a Master Micro-Technologist at Microbiologics, where he is responsible for helping customers understand why microorganisms behave the way they do. You could say he’s somewhat of a psychologist. Microbiologics has been lucky to have Stanley, a graduate of Gram-Positive Cocci University, as a member of their renowned Technical Support Team for over 20 years. Stanley says his favorite type of people are microbiologists and he enjoys traveling far and wide to meet them. Amazingly, Stanley has been on every continent – even Antarctica!
coccus is a Master Micro-Technologist at Microbiologics, where he is responsible for helping customers understand why microorganisms behave the way they do. You could say he’s somewhat of a psychologist. Microbiologics has been lucky to have Stanley, a graduate of Gram-Positive Cocci University, as a member of their renowned Technical Support Team for over 20 years. Stanley says his favorite type of people are microbiologists and he enjoys traveling far and wide to meet them. Amazingly, Stanley has been on every continent – even Antarctica!
How to submit inquiries: There are two ways to get Stanley’s help. You can email your questions to stanley@microbiologics.com or you can simply submit an inquiry from our Dear Stanley page. For urgent issues, please contact our Technical Support Team at techsupport@microbiolgics.com or 1.320.229.7045.
Read Next – 8 Best Practices for Growth Promotion Testing




Dear Stanley,
Using past data to establish the acceptance criteria has been met is a great option for solid media. What if you have a liquid media that requires visually comparable growth to that “previously obtained with a previously tested and approved batch of medium” and are unable to run parallel tests? Are documented pictures in procedures able to be used as comparable turbidity?
Arisa – Good question! Our first piece of advice is to reach out to your auditor for suggestions. Here are a few other recommendations:
– Check out our growth promotion testing section on our FAQ webpage: https://www.microbiologics.com/faq. There you’ll find a question and answer on what to do if you cannot perform parallel testing along with lots of other helpful tips.
– Go to USP <61>. This chapter states that liquid media is suitable if there is clearly visible growth. If you cannot test in parallel because you’re just starting GPT, then you may decide a photo is the next best option. Once the liquid media passes GPT, then it can be used in parallel with the next batch of media. You can read <61> here: https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c61.pdf
Please feel free to reach out to our Technical Support team at techsupport@microbiologics.com or 1.320.229.7045 with questions.