This post on external quality controls is the first in our series on Optimal QC in the Clinical Laboratory, which also covers third-party controls and IVD labelled controls.
By principle, quality control (QC) in the clinical laboratory must subject all instrumentation, assays, materials, and methods to external challenges that are removed, as much as possible, from the biases inherent to each analytical process. Quality control, by design, should add layers of removal from the assay to ensure the utmost degrees of objectivity. In fact, ISO 15189, used by laboratories around the world, states: “Laboratory shall use quality control materials that react to the examining system in a manner as close as possible to patient samples.”1 Other global standards organizations emphasize that quality control materials must be as similar as possible to patient samples, such as the Rili-BÄK in Germany.2 This blog post will define, compare, and contrast internal and external forms of quality control.
Many assays have integrated, built-in, or internal controls to demonstrate their operability at specific procedural steps. What role do these built-in controls play in quality control to assure precision and accuracy assessment of assays, personnel, and instruments? Can clinical laboratories rely on internal controls as a stand-alone for QC? Although internal controls are not new, these questions become increasingly important with more point-of-care (POC) assays and at-home tests coming to market. On typical at-home and POC tests, the internal controls feature prominently in the compact designs of the analytical devices, leading some users to assume that the internal controls satisfy the demands of QC. Additionally, these devices are sometimes used outside of clinical laboratories by personnel without the full training of a clinical laboratory professional. Therefore, education for their intended roles becomes even more important.
Internal controls serve useful roles as procedural controls in assays, but they are not designed to fulfill the requirements of QC for analytical accuracy and reliability by themselves. The use of external controls is critical, as only external controls monitor the entire assay from sample preparation to target detection. Thus, external controls challenge analytical procedures, instrumentation, and personnel similar to patient samples as defined in ISO 15189.
External Quality Control vs. External Quality Assessment (EQA)
Globally, the terms “external quality control” and “external quality assessment” (EQA) are used differently. Here we define these terms for the purpose of this blog. Neither external quality control nor EQA should be relied upon exclusively in a laboratory’s quality management system; both forms complement each other and are needed in laboratories.
In this blog, we use “external quality control” to reference controls tested on each run of an assay for precision and accuracy assessment of assays, personnel, and instruments. Any change in frequency must be determined by a laboratory’s individualized quality control plan.
We refer to “external quality assessment” interchangeably with proficiency schemes. The World Health Organization (WHO) defines EQA as “a system for objectively checking the laboratory’s performance using an external agency or facility.”3 A laboratory is compared to a source outside of their laboratory, to a peer group, or reference laboratory. EQA is used to ensure long term accuracy for peer comparison.
In summary, EQA testing forms the outermost layer of quality control, bringing in outside auditors to assess a laboratory’s analytical processes. For routine QC, external controls provide the degree of removal necessary to objectively monitor analytical performance on a more frequent basis.
What is an Internal Control?
An internal control is built into an assay often as reagents (e.g., nucleic acids, antibodies, etc.) integrated into a specific component of the assay.4 Internal controls may also be referred to as internal amplification controls, sample processing controls, or sample adequacy controls. Internal controls can be viewed as process controls – monitoring various stages of the procedure, but not challenging the entire assay or analytical process.
Internal controls react and provide amplification of a specific nucleic acid sequence regardless of the presence or absence of the analyte being tested. Internal controls are included to confirm DNA amplification, detect reagent failure, and detect amplification or specimen-associated inhibitory substances in a sample.5,6 Unless tested for, PCR inhibition can give rise to false negative results.
A signal should always be produced in an assay with an internal control, even if there is no target sequence present. In fact, if the internal processing control fails to meet acceptance criteria, the result of the specimen will often be reported as unresolved, invalid, or indeterminant rather than negative.
Figure 1. Generic example of a single-target at-home lateral flow antigen-based immunoassay to illustrate internal and external quality controls.
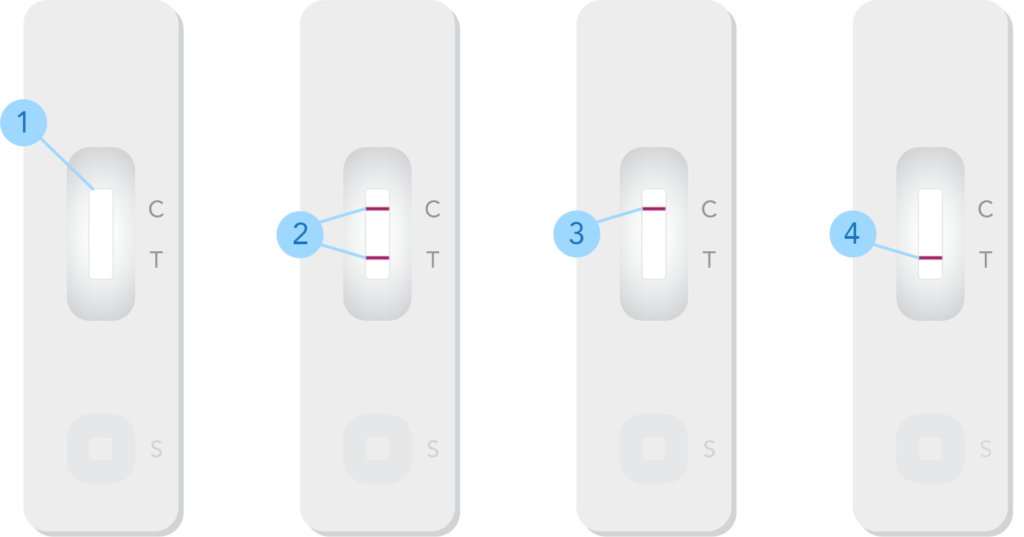
- Results window. The C represents the internal control. The T represents the test result. After the patient sample or external quality control is applied to the assay, bands (lines) appear next to the C and T to indicate positive, negative, or inconclusive results. A signal should always be produced for the internal control, even if there is no target sequence present.
- Positive result. Two lines appearing by the C and T indicate the assay has operated and detected the presence of the target antigen. If running an external positive control on the assay, this is the expected result.
- Negative result. A line appearing by the control area but not in the test area signifies that the assay did not detect the presence of the target antigen. If running an external negative control on the assay, this is the expected result.
- Invalid result. The absence of the control line indicates that the assay did not operate as intended; the test results cannot be interpreted as positive or negative. In the case of an internal control failure, the laboratory personnel should reference further instructions in their assay’s instructions for use (IFU).
Perhaps the simplest form of internal control to visualize is that of a single-target at-home lateral flow antigen-based immunoassay (see Figure 1 for generic assay example). Many of us became familiar with these at-home tests during the COVID-19 pandemic. The internal control is visualized by a line in the sample visualizer (labeled “C” above). In this example, the internal control contains reagents (e.g., antibodies, etc.) immobilized on a membrane to construct a test strip. If the test flows and the reagents work, the control line will appear. If the reagents do not work properly, the control line will not appear, and the assay should be called “indeterminant” and not negative. When the internal control fails to perform as expected, the clinical laboratory will need to follow the assay manufacturer’s instructions for next steps.
If the laboratory were using an external control, the laboratory personnel would use a known positive or negative control and run the sample similar to a patient sample. The laboratory personnel would verify the internal control worked properly and analyze that the positive or negative external control performed as expected on the assay. The result should appear in the “T” area of the result window. Thus, the external control evaluates the entire analytical process from sample preparation to target detection, while the internal control only verifies certain procedural steps in the assay. Again, the external control fulfills the criteria in ISO 15189 as the external control is processed in a similar manner as a patient sample.
Internal controls vary in style and complexity, like the assays for which they are used. As we reviewed above, they range from a single-target at-home-antigen based assays with internal antibody controls to a multiplex PCR assay using sample adequacy controls or internal amplification controls that are reported via an instrument system. In either case, internal controls are reagents built into an assay to ensure the instrument, reagents, and reactions are working properly.
Internal vs External Controls
Unlike external controls which must be handled and introduced into the assay, an internal control requires little to no action from the test administrator since internal controls are built into an assay or instrument.
Although useful as process controls, internal controls have their limitations. Since internal controls are often made of the same material as the assay calibrator or assay components, they may be biased to the assay. Additionally, internal controls are often not full process controls, which means they do not monitor each assay step of extraction, amplification, and detection. Thus, although internal controls are essential components of assays, external controls are necessary to provide a true challenge to the assay.
An external control must be handled and applied to the assay, much like a patient sample. Therefore, an external control monitors laboratory procedures and the technique of laboratory personnel, not just the operability of instrumentation. By challenging every step of the analytical procedure, an external control serves as a true process control.
The Proper Role of Internal Controls
As mentioned, internal controls provide a useful indicator for an assay’s status, signifying that the assay’s components are working properly and that the assay is ready for detection. In certain settings, like point-of-care testing, this indicator can serve as an important step in the analytical process, while not fulfilling the demands of QC. As outlined above, ISO 15189 highlights the importance of quality controls challenging the entire analytical process similar to patient samples. Thus, clinical laboratory professionals should not rely on internal controls alone and should always run external controls to challenge all steps of the analytical process.
Types of External Controls
We understand the advantage of using external controls as a true challenge to the analytical process; however, there are many different forms of external controls and not all are created equal.2 Below, we outline and define the types of external quality control. The next blog in our series will feature the optimal form of external controls, third-party QC.
Independent or Third-Party controls are the gold standard in external quality control material as they are produced by an independent, third-party manufacturer. Independent, third-party controls provide an unbiased, independent assessment of analytical performance.
Assay Manufacturer Made Controls are another form of external quality control. These materials are provided by an assay manufacturer often within a test kit. Similar to internal controls, assay manufacturer made controls are often made of the same reagents as assay calibrators and may introduce bias into analytical performance. We will evaluate the risk associated with reliance on assay manufacturer made controls in a case study presented in the next blog post in this series.
Lab-Developed Quality Control materials refer to the process of a laboratory choosing to develop their own quality control materials by obtaining reference materials themselves. This process often requires extensive evaluation or characterization of the materials on part of the laboratory.
The final form of external control material that we will review is the use of patient samples. A laboratory may choose to use an existing patient specimen for quality control testing. Like laboratory-developed QC, use of patient samples often requires extensive evaluation or characterization of materials. Laboratories may also need to check with local regulations to see if patient samples are an acceptable form of QC in their regions. Another limitation to reliance on patient samples as an external form of QC is continuity of supply. There is not a guarantee that the clinical laboratory will have adequate access to positive patient samples.
| Internal Controls | External Controls | |||
|
Components of an assay used to ensure instrument / reagents / reactions are working properly. Not designed to be a full process control. Although internal controls are essential components of assays, internal controls should be used alongside external controls to challenge the assay and sampling process. |
Independent/Third-Party | Assay Manufacturer Made | Lab Developed | Patient Samples |
|
Controls produced by an independent, third-party manufacturer. An ideal form of quality control as these materials provide an unbiased, independent assessment of analytical performance. |
Manufactured and provided by an assay manufacturer sold with test kit or separately. Often made of the same material as assay calibrators and may introduce bias to analytical performance. |
A lab may choose to obtain reference materials to develop their own quality control material. Often requires extensive evaluation or characterization on part of the lab. |
A lab may use an existing patient specimen for quality control testing. Like lab-developed QC, it often requires extensive evaluation or characterization. Lab may need to check with local regulations to see if patient samples are an acceptable form of QC. |
|
Optimal QC: External, Third-Party, IVD
External controls form the foundation of optimal QC. In addition to being external to the assay, QC products should be third-party and IVD.
Why Microbiologics
Microbiologics maximizes the advantages of external controls by engineering QC products to resemble patient samples in the manner of sample collection and handling. Available in lyophilized swab and pellet formats, our molecular controls are designed to be processed like patient samples, challenging every step of the analytical procedure, from sample handling to detection. We offer a broad portfolio of controls for the major syndromic groups of infectious disease.
View molecular controls.
Sources
1. ISO 15189:2012. Medical laboratories – requirements for quality and competence. Clause 5.6.2.2 Quality Control Materials.
2. Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations (Rili-BÄK). J Lab Med 2015; 31:26-69. Clause B1, 2.1.1 Procedure (5).
3 World Health Organization. (2009, November 2). Content sheet 10-1: Overview of external quality assessment (EQA). https://cdn.who.int/media/docs/default-source/essential-medicines/norms-and-standards/10-b-eqa-contents.pdf?sfvrsn=181d9a32_4&download=true
4. Brunstein, J. (2013, November 18). Process controls: Internal and External .Medical Laboratory Observer. https://www.mlo-online.com/home/article/13005863/process-controls-internal-and-external
5. Kavlick MF. Development of a universal internal positive control. Biotechniques. 2018 Nov;65(5):275-280. doi: 10.2144/btn-2018-0034. PMID: 30394127.
6. Hoorfar J, Malorny B, Abdulmawjood A, Cook N, Wagner M, Fach P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J Clin Microbiol. 2004 May;42(5):1863-8. doi: 10.1128/JCM.42.5.1863-1868.2004. PMID: 15131141; PMCID: PMC404670.



0 Comments